
Get the free Louis W - fda
Show details
DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION DISTRICT ADDRESS AND PHONE NUMBER DATE(S) OF INSPECTION 300 River Place, Suite 5900 Detroit, MI 48207 (313) 393-8100 Fax:(313)
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign
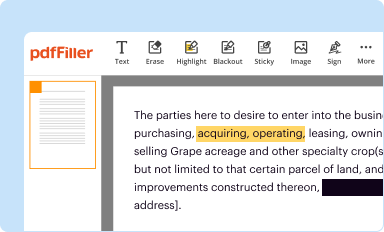
Edit your louis w - fda form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
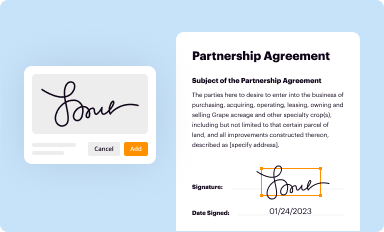
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your louis w - fda form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing louis w - fda online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Check your account. In case you're new, it's time to start your free trial.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit louis w - fda. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. You may try it out for yourself by signing up for an account.
How to fill out louis w - fda
How to fill out louis w - fda:
01
Begin by gathering all relevant information and documents required for the louis w - fda form.
02
Carefully read the instructions provided on the form to ensure you understand the information being asked for.
03
Start by entering your personal details accurately, including your full name, contact information, and any identification numbers if required.
04
Provide any additional information requested, such as your current employment details, educational background, or medical history, depending on the nature of the form.
05
Double-check all the entered information to ensure it is accurate and error-free.
06
If applicable, attach any supporting documentation required, such as identification cards, proof of address, or medical certificates.
07
Review the completed form one last time to make sure you haven't missed any sections or made any mistakes.
08
Sign and date the form as instructed.
09
If necessary, submit the form through the designated method, such as mailing it to the provided address or submitting it online.
Who needs louis w - fda:
01
Individuals who are involved in the FDA (Food and Drug Administration) application or evaluation process may require louis w - fda.
02
It could be individuals seeking approvals, authorizations, or certifications related to food or drug products.
03
Manufacturers, distributors, importers, or exporters of food or drug products may need to fill out louis w - fda.
04
Researchers or individuals involved in clinical trials or studies that require FDA compliance might also require this form.
05
It is recommended to verify specific requirements and guidelines to determine who exactly needs to fill out louis w - fda in a given situation.
Fill form : Try Risk Free
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is louis w - fda?
Louis W - FDA refers to the Food and Drug Administration (FDA) regulations related to the reporting of adverse events for drugs and medical devices.
Who is required to file louis w - fda?
Manufacturers, distributors, and importers of drugs and medical devices are required to file adverse event reports under Louis W - FDA.
How to fill out louis w - fda?
To fill out Louis W - FDA reports, companies must provide detailed information about adverse events, including product details, patient information, and the nature of the event.
What is the purpose of louis w - fda?
The purpose of Louis W - FDA is to monitor and ensure the safety of drugs and medical devices by tracking adverse events and taking appropriate regulatory actions if necessary.
What information must be reported on louis w - fda?
The information that must be reported on Louis W - FDA includes product details, such as brand name, manufacturer, lot number, and expiration date, as well as patient details, adverse event description, and any medical interventions.
When is the deadline to file louis w - fda in 2023?
The deadline to file Louis W - FDA reports in 2023 may vary depending on the specific reporting requirements and regulations. It is recommended to refer to the FDA's official guidelines and deadlines for accurate information.
What is the penalty for the late filing of louis w - fda?
The penalty for the late filing of Louis W - FDA reports can vary and may include fines, warning letters, or other regulatory actions imposed by the FDA. The specific penalties depend on the severity of the violation and the circumstances surrounding the late filing.
Where do I find louis w - fda?
The premium version of pdfFiller gives you access to a huge library of fillable forms (more than 25 million fillable templates). You can download, fill out, print, and sign them all. State-specific louis w - fda and other forms will be easy to find in the library. Find the template you need and use advanced editing tools to make it your own.
Can I edit louis w - fda on an iOS device?
Use the pdfFiller app for iOS to make, edit, and share louis w - fda from your phone. Apple's store will have it up and running in no time. It's possible to get a free trial and choose a subscription plan that fits your needs.
How do I complete louis w - fda on an iOS device?
Get and install the pdfFiller application for iOS. Next, open the app and log in or create an account to get access to all of the solution’s editing features. To open your louis w - fda, upload it from your device or cloud storage, or enter the document URL. After you complete all of the required fields within the document and eSign it (if that is needed), you can save it or share it with others.
Fill out your louis w - fda online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Not the form you were looking for?
Keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.





















