Impact: Changes: — All animals treated with PROPOSAL1PC65 must undergo the clinical trial of such medicine, as described in this new chapter.
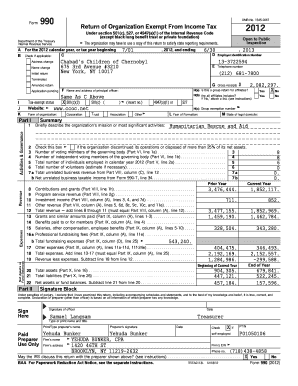
SUMMARY: The proposed rule addresses the requirement that animal drugs be registered under the Federal Food, Drug, and Cosmetic Act (FDA) before the drug can be marketed in the United States. The regulatory proposal revises, updates, or eliminates the requirement of a “biologic drug product application” to register animal drugs for human use prior to their marketing. It also revises, updates, or eliminates the requirement for animal drugs to undergo clinical trials in the United States prior to marketing.
The regulatory proposal is the first of three proposals under FDA jurisdiction that address the registration and labeling requirements for animal drugs. The proposed rule would have the greatest impact on the following drugs (see FDA's Drugs — Animal Testing : Overview for a list of animal drugs, along with the FDA's Animal Drugs — List of List-Only Animals and the FDA's Food Animal Drug Notification Form, for more information): Pfizer's Native in oral form, including products marketed to treat spasticity; Eli Lilly's Lyrics in rectal formulation; Novartis' Physio in the ear and nasal mucosa; Bringer Ingelheim's Fountain in oral form, including products marketed to treat spasticity and the oropharynx; Baxter's Biodesign in rectal, subcutaneous, and injectable forms and in oral, subcutaneous, and injectable form; Toronto in oral form; Bovine Alopecia, an animal drug used in the management of testosterone deficiency; Merck's Enamel in the vagina and male urethra; AstraZeneca's Ciprofloxacin in the rectal and recto vaginal route; and Lilly's Zofran in rectal and recto vaginal route. The proposed rule also addresses whether a drug product must be included on the FDA's “Priority List” under the FDA's ``New Animal Drug Program,'' by re-categorizing some animal drugs on the Priority List.

Get the free 71 fr 51276 august 29 2006 form - gpo
Show details
51276 Federal Register / Vol. 71 No. 167 / Tuesday August 29 2006 / Proposed Rules DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Parts 20 201 207 314 330 514 515 601 607 610 and 1271 Docket No. 2005N 0403 RIN 0910 AA49 Requirements for Foreign and Domestic Establishment Registration and Listing for Human Drugs Including Drugs that are Regulated Under a Biologics License Application and Animal Drugs AGENCY HHS. jlentini on PROD1PC65 with PROPOSAL2 ACTION Proposed...
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign
Edit your 71 fr 51276 august form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your 71 fr 51276 august form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit 71 fr 51276 august online
To use the professional PDF editor, follow these steps:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit 71 fr 51276 august. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Fill form : Try Risk Free
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is 71 fr 51276 august?
71 fr 51276 august is a regulation issued by the Federal Register on August 1, 2021.
Who is required to file 71 fr 51276 august?
The individuals or entities specified in the regulation are required to file 71 fr 51276 august.
How to fill out 71 fr 51276 august?
To fill out 71 fr 51276 august, you need to follow the instructions provided in the regulation. It may involve gathering and reporting specific information as outlined in the document.
What is the purpose of 71 fr 51276 august?
The purpose of 71 fr 51276 august is to implement certain rules or requirements as determined by the issuing authority for the relevant subject matter.
What information must be reported on 71 fr 51276 august?
The specific information that must be reported on 71 fr 51276 august will be detailed within the regulation itself. It may vary depending on the nature of the requirements.
When is the deadline to file 71 fr 51276 august in 2023?
The specific deadline to file 71 fr 51276 august in 2023 will be specified in the regulation or any subsequent updates or amendments.
What is the penalty for the late filing of 71 fr 51276 august?
The penalty for late filing of 71 fr 51276 august will be stated in the regulation or any applicable laws or regulations governing such penalties.
How do I modify my 71 fr 51276 august in Gmail?
71 fr 51276 august and other documents can be changed, filled out, and signed right in your Gmail inbox. You can use pdfFiller's add-on to do this, as well as other things. When you go to Google Workspace, you can find pdfFiller for Gmail. You should use the time you spend dealing with your documents and eSignatures for more important things, like going to the gym or going to the dentist.
How can I edit 71 fr 51276 august from Google Drive?
Using pdfFiller with Google Docs allows you to create, amend, and sign documents straight from your Google Drive. The add-on turns your 71 fr 51276 august into a dynamic fillable form that you can manage and eSign from anywhere.
How can I send 71 fr 51276 august for eSignature?
To distribute your 71 fr 51276 august, simply send it to others and receive the eSigned document back instantly. Post or email a PDF that you've notarized online. Doing so requires never leaving your account.
Fill out your 71 fr 51276 august online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Not the form you were looking for?
Keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.