Get the free Controlled Substance Receipt Record
Show details
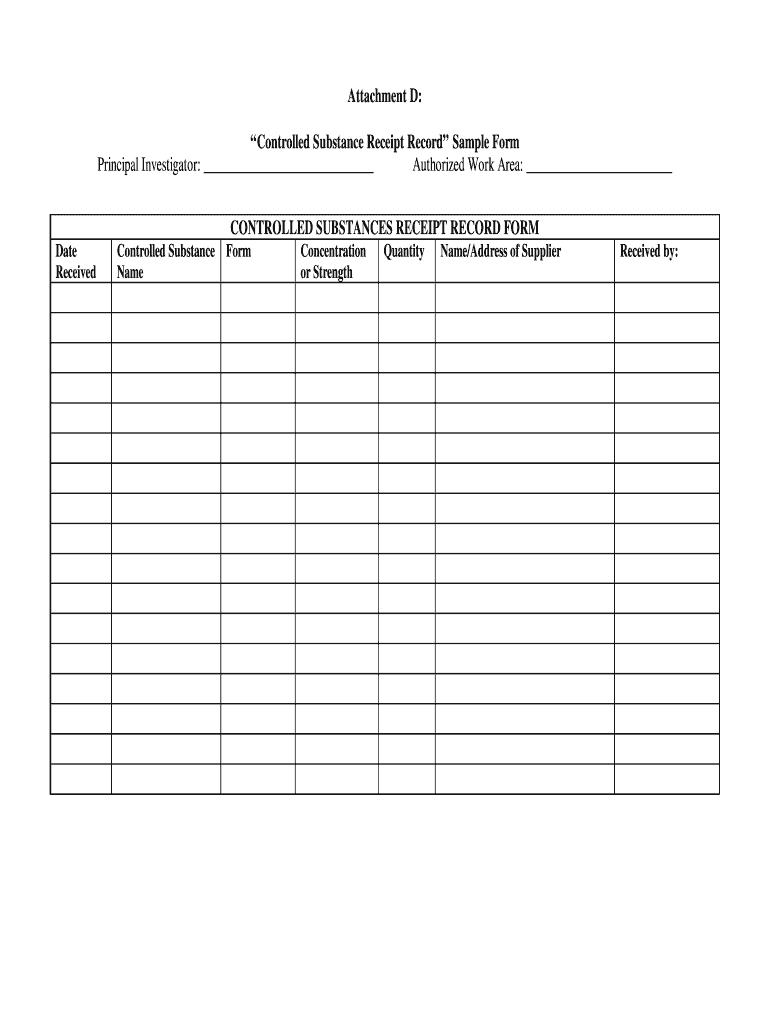
This document serves as a template for recording the receipt of controlled substances, including details such as the name of the substance, its concentration, quantity, supplier information, and the
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign controlled substance receipt record

Edit your controlled substance receipt record form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your controlled substance receipt record form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit controlled substance receipt record online
Follow the guidelines below to benefit from a competent PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit controlled substance receipt record. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out controlled substance receipt record

How to fill out Controlled Substance Receipt Record
01
Obtain a Controlled Substance Receipt Record form from your facility.
02
Fill in the date of receipt at the top of the form.
03
Enter the name of the controlled substance being received.
04
Include the quantity of the controlled substance received.
05
Document the name and address of the supplier or manufacturer from whom the substance was received.
06
Record the lot number and expiration date if applicable.
07
Sign and print your name as the receiving individual.
08
Have the form witnessed by a second authorized person if required by your facility's policy.
09
Submit the completed form to the designated department for record-keeping.
Who needs Controlled Substance Receipt Record?
01
Healthcare professionals handling controlled substances.
02
Pharmacists involved in receiving shipments of controlled drugs.
03
Medical institutions that need to document inventory for regulatory compliance.
04
Researchers who need to account for the receipt of controlled substances.
Fill
form
: Try Risk Free






People Also Ask about
What is a controlled substance in English?
A drug or other substance that is tightly controlled by the government because it may be abused or cause addiction. The control applies to the way the substance is made, used, handled, stored, and distributed. Controlled substances include opioids, stimulants, depressants, hallucinogens, and anabolic steroids.
What is recorded in the controlled drugs Record Book?
Controlled drug registers. You must record any movement of a Schedule 2 controlled drug in a controlled drugs register. The register should be a bound book with numbered pages. You must use the register to record the receipt, administration, disposal and transfer of controlled drugs held by the care home.
What is the difference between a sales document and a purchase document?
Basically, a sales order is an agreement to purchase goods or services for a specific period of time, and a purchase order is an agreement to supply goods or services for a specific period of time. The main difference between a sales order and a purchase order is the term of the contract.
What should you document with a controlled substance?
When transferring controlled substances from one pharmacy to another, a pharmacist must document the name, dosage, quantity, and strength of any drug being transferred. They must also document the date of transfer along with addresses and DEA registration numbers of the pharmacies.
What is the difference between sales and purchase?
Purchase is a process through which a person gets the ownership of some goods or properties transferred in his name from another, on payment of money. Similarly, sale is a process through which the ownership of some goods or properties is transferred from one person (seller) to another person (buyer), for a price.
What is a sales and purchase record?
You can use the sales journal to record all revenue you generate from the products or services you provide. You can use a purchase journal to record items you bought for your business, or inventory to make the products you sell.
What is the difference between a sales order and a purchase order?
Purchase orders are used by buyers to initiate the purchasing process with a supplier. Sales orders are sent by suppliers to buyers after receiving a purchase order from the buyer - verifying details and the confirmation of the purchase.
What is the difference between a purchase record and a sales record?
Examining each document's purpose — purchase orders track what your company buys from vendors, while sales orders record what customers buy from you — will clarify when to use each one. Both documents play complementary roles in successful transactions, connecting buyers and sellers through well-defined processes.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Controlled Substance Receipt Record?
The Controlled Substance Receipt Record is a document used to track and record the receipt of controlled substances by organizations that handle such materials, ensuring compliance with regulatory requirements.
Who is required to file Controlled Substance Receipt Record?
Entities that handle controlled substances, such as pharmacies, hospitals, and research facilities, are required to file a Controlled Substance Receipt Record.
How to fill out Controlled Substance Receipt Record?
To fill out a Controlled Substance Receipt Record, one must enter details such as the date of receipt, name of the substance, quantity received, supplier information, and any relevant batch or lot numbers.
What is the purpose of Controlled Substance Receipt Record?
The purpose of the Controlled Substance Receipt Record is to maintain an accurate inventory, ensure legal compliance, and facilitate accountability in the handling of controlled substances.
What information must be reported on Controlled Substance Receipt Record?
The information that must be reported includes the date of receipt, the name and schedule of the controlled substance, quantities received, supplier details, and any additional relevant documentation or identifiers.
Fill out your controlled substance receipt record online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Controlled Substance Receipt Record is not the form you're looking for?Search for another form here.
Relevant keywords
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















