Get the free Continuing Review of an Existing Animal Care and Use Protocol - uis
Show details
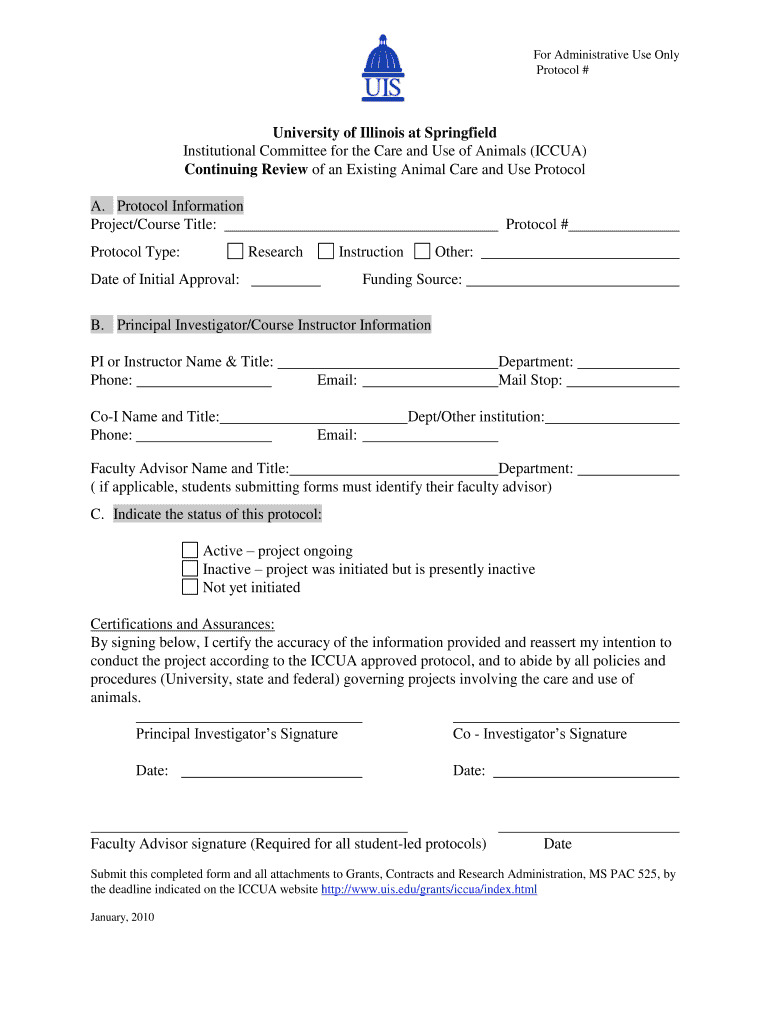
For Administrative Use Only Protocol # University of Illinois at Springfield Institutional Committee for the Care and Use of Animals (ICCA) Continuing Review of an Existing Animal Care and Use Protocol
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign continuing review of an

Edit your continuing review of an form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your continuing review of an form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit continuing review of an online
In order to make advantage of the professional PDF editor, follow these steps:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit continuing review of an. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out continuing review of an

How to Fill Out Continuing Review of an:
01
Start by reviewing the instructions provided by your institution or the regulatory body overseeing the review process. It is important to familiarize yourself with the specific requirements and guidelines to ensure that your review is completed accurately and efficiently.
02
Gather all relevant documents and information related to the study or project. This may include previous approval letters, protocol amendments, participant information sheets, consent forms, and any adverse event reports or changes made since the initial approval.
03
Begin by completing the cover page of the continuing review form. This typically includes basic information such as the title of the study, principal investigator's name, contact information, and the date of the review.
04
Provide a summary of the progress of the study or project since the last review. Highlight any significant milestones, recruitment updates, changes in personnel, or modifications made to the study protocol. Be thorough and concise in your descriptions.
05
Evaluate the overall risk level associated with the study. Assess whether there have been any adverse events, unanticipated problems, or deviations from the approved protocol during the review period. It is crucial to document any risks or issues and provide a plan for mitigation or corrective actions if necessary.
06
Consider whether any changes to the study design, procedures, or informed consent process are required. If so, provide a detailed description of the proposed changes and the rationale behind them. This may include updates to recruitment strategies, data collection methods, or participant eligibility criteria.
07
Review the status of informed consent for all participants and ensure that necessary documentation is on file. Confirm that participants have given their informed consent, understand the nature of the study, and are aware of any potential risks or benefits. This is a critical aspect of ensuring ethical conduct throughout the research or project.
08
Complete any additional sections or questions included in the continuing review form, such as those related to financial conflicts of interest or updates on research funding.
Who Needs Continuing Review of an:
01
Researchers or investigators conducting studies involving human subjects typically need to undergo a continuing review process. This ensures that ethical standards are maintained, participant safety is prioritized, and any necessary modifications or updates are evaluated.
02
Institutional review boards (IRBs) or ethics committees are responsible for overseeing the continuing review of studies. They ensure that the research complies with applicable laws, regulations, and institutional policies. IRBs play a vital role in protecting the rights and welfare of human subjects involved in research.
03
Sponsors or funding agencies may also require researchers to undergo continuing review as part of the ongoing monitoring and assessment process. This helps to ensure that the research remains within the scope of the original approved protocol and that any adverse events or risks are promptly addressed.
Overall, the continuing review process serves as a mechanism for ongoing evaluation and oversight, promoting the responsible conduct of research and the protection of human subjects' rights and welfare.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I execute continuing review of an online?
pdfFiller has made it easy to fill out and sign continuing review of an. You can use the solution to change and move PDF content, add fields that can be filled in, and sign the document electronically. Start a free trial of pdfFiller, the best tool for editing and filling in documents.
How can I edit continuing review of an on a smartphone?
You may do so effortlessly with pdfFiller's iOS and Android apps, which are available in the Apple Store and Google Play Store, respectively. You may also obtain the program from our website: https://edit-pdf-ios-android.pdffiller.com/. Open the application, sign in, and begin editing continuing review of an right away.
How do I edit continuing review of an on an Android device?
You can edit, sign, and distribute continuing review of an on your mobile device from anywhere using the pdfFiller mobile app for Android; all you need is an internet connection. Download the app and begin streamlining your document workflow from anywhere.
Fill out your continuing review of an online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Continuing Review Of An is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















