Get the Certification for a Drug-Free Workplace - abqha
Show details
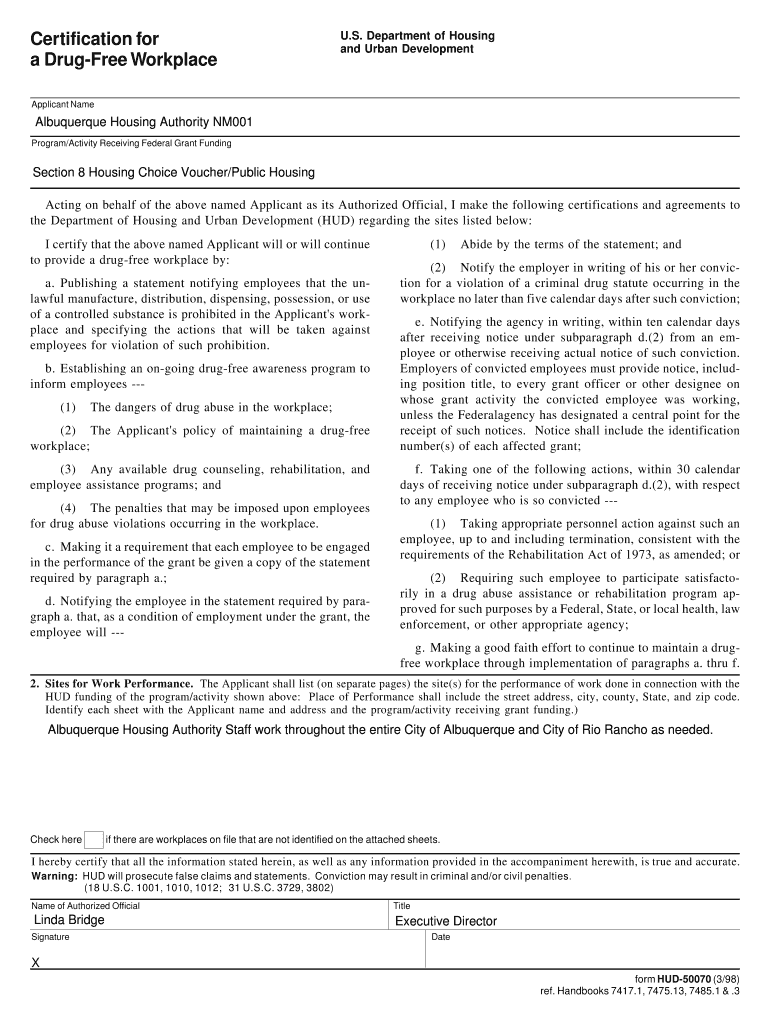
Certification for a Degree Workplace U.S. Department of Housing and Urban Development Applicant Name Albuquerque Housing Authority NM001 Program/Activity Receiving Federal Grant Funding Section 8
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign certification for a drug

Edit your certification for a drug form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your certification for a drug form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing certification for a drug online
Follow the guidelines below to benefit from a competent PDF editor:
1
Check your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit certification for a drug. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
Dealing with documents is simple using pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out certification for a drug

How to fill out certification for a drug?
01
Start by gathering all the necessary information and documents required for the certification process. This may include the drug's name, ingredients, manufacturing details, and any related clinical trial data.
02
Begin the certification form by providing your personal information, such as your name, contact details, and professional credentials. Ensure that all the information is accurate and up to date.
03
Proceed by entering the details of the drug for which you are seeking certification. This may include the drug's generic and brand names, dosage form, strength, and intended use.
04
If applicable, provide information about the manufacturing facility and any Good Manufacturing Practices (GMP) compliance certificates associated with it. Include details about the facility's location, staff qualifications, and quality control procedures.
05
Describe the drug's intended use and target patient population. Specify any specific conditions or diseases that the drug is designed to treat, manage, or prevent.
06
If there are any clinical trial results or studies supporting the drug's safety and efficacy, include them in a separate section. Provide details about the study design, sample size, methodology, and the outcomes observed.
07
Include any additional information or documentation that is required by the certification authority. This may include safety data, stability studies, labeling information, or any adverse events associated with the drug.
08
Review the completed certification form to ensure accuracy and completeness. Double-check all the information provided and make any necessary corrections before submitting the form.
09
Finally, submit the certification form along with any required fees to the relevant certification authority or regulatory body. Follow up with the authority to track the progress of your certification application.
Who needs certification for a drug?
01
Drug manufacturers: Any pharmaceutical company or individual involved in the production and distribution of drugs needs certification to ensure compliance with regulatory standards and demonstrate the safety and efficacy of their products.
02
Healthcare professionals: Health practitioners who prescribe, dispense, or administer drugs may need certification to ensure that they are following the appropriate guidelines and regulations related to drug usage and patient safety.
03
Regulatory authorities: Government agencies responsible for overseeing the safety, quality, and efficacy of drugs often require certification as part of their regulatory processes. This helps ensure that only safe and effective drugs are available on the market.
04
Research institutions: Institutions involved in drug research and development may need certification to demonstrate the quality and safety of their experimental drugs during the clinical trial phase.
05
Pharmacists and pharmacies: Pharmacies and pharmacists dispensing drugs to patients need certification to ensure proper handling, storage, and distribution of medications, thereby safeguarding patient health and well-being.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an electronic signature for the certification for a drug in Chrome?
Yes. With pdfFiller for Chrome, you can eSign documents and utilize the PDF editor all in one spot. Create a legally enforceable eSignature by sketching, typing, or uploading a handwritten signature image. You may eSign your certification for a drug in seconds.
Can I create an electronic signature for signing my certification for a drug in Gmail?
It's easy to make your eSignature with pdfFiller, and then you can sign your certification for a drug right from your Gmail inbox with the help of pdfFiller's add-on for Gmail. This is a very important point: You must sign up for an account so that you can save your signatures and signed documents.
Can I edit certification for a drug on an Android device?
You can edit, sign, and distribute certification for a drug on your mobile device from anywhere using the pdfFiller mobile app for Android; all you need is an internet connection. Download the app and begin streamlining your document workflow from anywhere.
What is certification for a drug?
Certification for a drug is a document that ensures the drug meets certain quality and safety standards set by regulatory authorities.
Who is required to file certification for a drug?
The manufacturer or distributor of the drug is required to file certification for a drug.
How to fill out certification for a drug?
To fill out certification for a drug, the manufacturer or distributor must provide detailed information about the drug, its ingredients, production process, and quality control measures.
What is the purpose of certification for a drug?
The purpose of certification for a drug is to ensure that the drug is safe, effective, and meets regulatory requirements before it is marketed and sold to the public.
What information must be reported on certification for a drug?
The information that must be reported on certification for a drug includes details about the drug's composition, manufacturing process, quality control measures, and any potential risks or side effects.
Fill out your certification for a drug online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Certification For A Drug is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















