Get the free Cord bPilotb Trial - University of Nottingham - nottingham ac
Show details
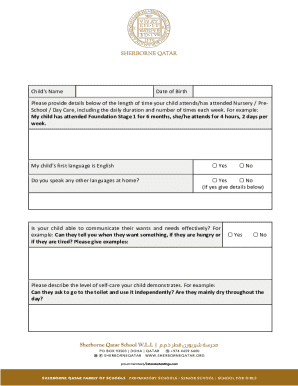
Cord Pilot Trial Immediate cord clamping versus deferred cord clamping for preterm birth before 32 weeks gestation: a pilot randomized trial Final Version 4 dated 14 May 2014 MAIN SPONSOR: Nottingham
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign cord bpilotb trial

Edit your cord bpilotb trial form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your cord bpilotb trial form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit cord bpilotb trial online
Use the instructions below to start using our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit cord bpilotb trial. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Create an account to find out for yourself how it works!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out cord bpilotb trial

How to fill out cord bpilotb trial:
01
Start by gathering all the necessary documents and information required for the trial.
02
Carefully read and understand the instructions provided for filling out the trial form.
03
Ensure that you have a clear understanding of the purpose and objective of the cord bpilotb trial.
04
Begin by providing your personal details accurately, such as your name, contact information, and other required identification details.
05
Follow the specified format and guidelines for providing any medical or health-related information that may be required in the trial form.
06
Double-check all the information filled in the trial form for any errors or discrepancies before submitting it.
07
If there are any questions or sections in the trial form that you are unsure about, consult the provided guidelines or seek assistance from the trial coordinator.
08
Once you have completed filling out the trial form, make sure to review it one last time to ensure that all the necessary information is provided.
09
Finally, submit the filled-out cord bpilotb trial form according to the specified instructions and within the designated deadline.
Who needs cord bpilotb trial:
01
Individuals who are interested in exploring new medical treatments or therapies for certain conditions or diseases may consider participating in the cord bpilotb trial.
02
Patients who have exhausted traditional treatment options and are willing to try experimental or investigational treatments may find the cord bpilotb trial beneficial.
03
Medical professionals and researchers who aim to understand the effectiveness and potential benefits of cord bpilotb in treating specific conditions may require participants for the trial.
04
Regulatory authorities and government agencies that oversee and evaluate new medical interventions may require a certain number of participants in the cord bpilotb trial to assess its safety and efficacy.
05
Organizations or companies involved in the development and production of cord bpilotb may recruit participants for the trial to gather data and evidence for obtaining regulatory approvals and marketing authorizations.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify cord bpilotb trial without leaving Google Drive?
Using pdfFiller with Google Docs allows you to create, amend, and sign documents straight from your Google Drive. The add-on turns your cord bpilotb trial into a dynamic fillable form that you can manage and eSign from anywhere.
How can I fill out cord bpilotb trial on an iOS device?
Get and install the pdfFiller application for iOS. Next, open the app and log in or create an account to get access to all of the solution’s editing features. To open your cord bpilotb trial, upload it from your device or cloud storage, or enter the document URL. After you complete all of the required fields within the document and eSign it (if that is needed), you can save it or share it with others.
How do I edit cord bpilotb trial on an Android device?
Yes, you can. With the pdfFiller mobile app for Android, you can edit, sign, and share cord bpilotb trial on your mobile device from any location; only an internet connection is needed. Get the app and start to streamline your document workflow from anywhere.
What is cord bpilotb trial?
Cord bpilotb trial is a trial program designed to test the effectiveness of a new treatment or medication.
Who is required to file cord bpilotb trial?
Medical researchers and pharmaceutical companies are required to file cord bpilotb trial.
How to fill out cord bpilotb trial?
Cord bpilotb trial can be filled out by submitting relevant data and information on the designated platform or database.
What is the purpose of cord bpilotb trial?
The purpose of cord bpilotb trial is to gather data on the safety and efficacy of a new treatment or medication.
What information must be reported on cord bpilotb trial?
Information such as patient demographics, treatment protocols, adverse events, and outcome measures must be reported on cord bpilotb trial.
Fill out your cord bpilotb trial online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Cord Bpilotb Trial is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.