Get the free Continuing Review Report - Crescent City IRB
Show details
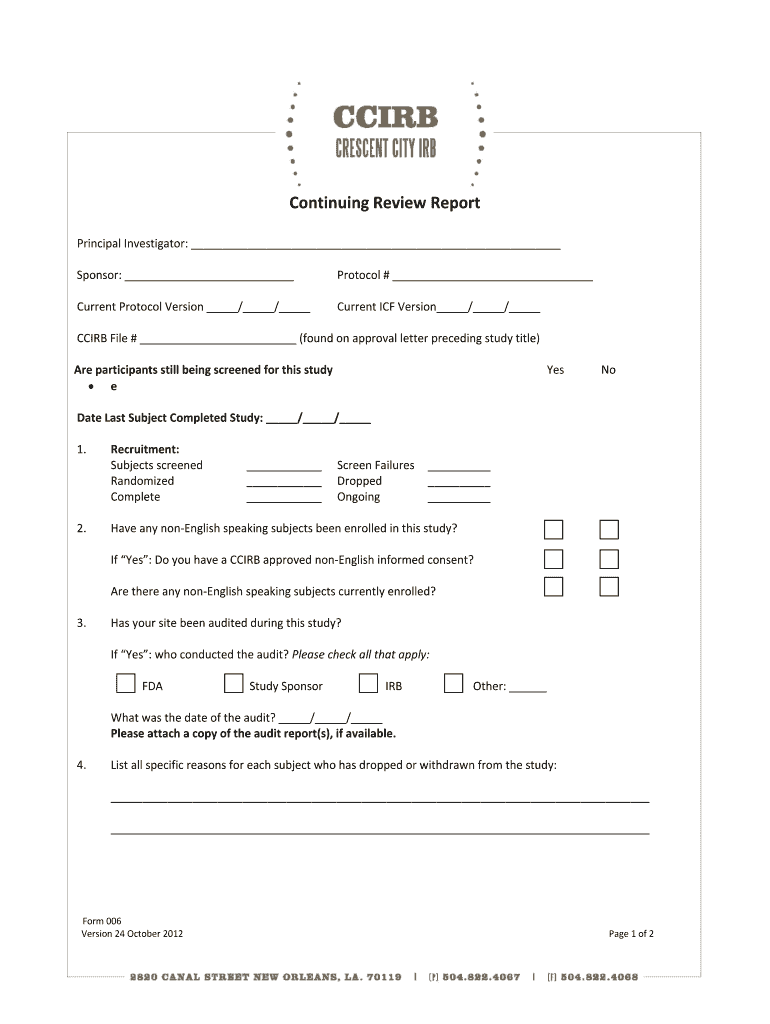
ContinuingReviewReport PrincipalInvestigator: Sponsor: Protocol# CurrentProtocolVersion / / CurrentICFVersion / / CCIRBFile# (foundonapprovalletterprecedingstudytitle) Areparticipantsstillbeingscreenedforthisstudy
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign continuing review report
Edit your continuing review report form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your continuing review report form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit continuing review report online
To use our professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit continuing review report. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
It's easier to work with documents with pdfFiller than you could have believed. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out continuing review report
How to fill out a continuing review report:
01
Start by gathering all the necessary information and documents related to the study that is being reviewed. This may include previous reports, study protocols, consent forms, participant data, and any updates or changes made to the study since the last review.
02
Familiarize yourself with the specific requirements and guidelines set forth by the institutional review board (IRB) or any other regulatory body overseeing the study. This will ensure that you are following the correct format and including all the necessary information in the report.
03
Begin the continuing review report by providing a brief overview of the study, including the research question or objective, study design, participant population, and any relevant background information. This section should give a clear and concise overview of the study to provide context for the subsequent sections.
04
Next, provide a detailed summary of the study progress since the last review. This may include recruitment updates, participant enrollment and retention rates, any adverse events or unanticipated problems that have occurred, protocol deviations, changes to the study procedures, or any other relevant information. Be sure to include accurate and up-to-date data to support your summary.
05
Discuss any modifications or amendments made to the study since the last review. This may include changes to the study protocol, consent forms, study procedures, or any other modifications that have been approved by the IRB. Clearly state the rationale for these changes and explain how they have been implemented.
06
Evaluate and discuss the risks and benefits of the study based on the data and information provided. This may include an assessment of any potential harm or discomfort to the participants, the potential for scientific or societal benefits, and any measures taken to minimize risks or enhance benefits. It is important to effectively communicate the ethical considerations and safeguards in place to protect the rights and welfare of the participants.
Who needs a continuing review report:
01
Researchers and investigators conducting human subjects research studies.
02
Institutions or organizations that have an institutional review board (IRB) or an ethics committee responsible for overseeing research involving human participants.
03
Regulatory bodies or agencies that require regular updates and reports on ongoing research studies to ensure compliance with ethical and legal standards.
Ultimately, the need for a continuing review report arises from the ethical principle of ongoing evaluation and oversight of research involving human participants. This ensures that studies are conducted in a manner that protects the rights, safety, and welfare of the participants, and that the research remains scientifically valid and relevant.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find continuing review report?
It's simple using pdfFiller, an online document management tool. Use our huge online form collection (over 25M fillable forms) to quickly discover the continuing review report. Open it immediately and start altering it with sophisticated capabilities.
How can I edit continuing review report on a smartphone?
The easiest way to edit documents on a mobile device is using pdfFiller’s mobile-native apps for iOS and Android. You can download those from the Apple Store and Google Play, respectively. You can learn more about the apps here. Install and log in to the application to start editing continuing review report.
How do I fill out continuing review report using my mobile device?
Use the pdfFiller mobile app to complete and sign continuing review report on your mobile device. Visit our web page (https://edit-pdf-ios-android.pdffiller.com/) to learn more about our mobile applications, the capabilities you’ll have access to, and the steps to take to get up and running.
What is continuing review report?
The continuing review report is a process in research studies that ensures the ongoing ethical and regulatory compliance of the study.
Who is required to file continuing review report?
Principal investigators and research teams are typically required to file continuing review reports for their studies.
How to fill out continuing review report?
Continuing review reports are usually completed by providing updated information on study progress, participant safety, and regulatory compliance.
What is the purpose of continuing review report?
The purpose of the continuing review report is to ensure that ongoing research studies adhere to ethical standards, participant safety, and regulatory requirements.
What information must be reported on continuing review report?
Information such as study progress, adverse events, protocol deviations, and changes to study procedures must be reported on the continuing review report.
Fill out your continuing review report online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Continuing Review Report is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.