
Get the free Continuing Review Form - navrongo-hrc
Show details
SARONG HEALTH RESEARCH Center
INSTITUTIONAL REVIEW BOARD
P. O BOX 114
SARONG U/E REGION, GHANA
IRB×sarong.sitcom.org
Tel: (233) (0)3821 22348, Fax: (233) (0)3821 22320
CONTINUING REVIEW SUBMISSION
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign continuing review form
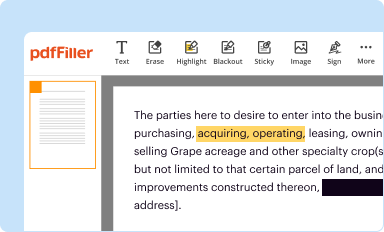
Edit your continuing review form form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
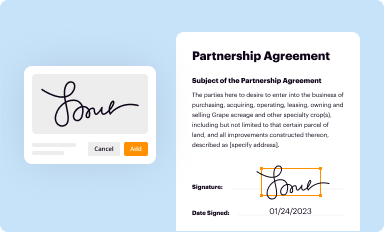
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your continuing review form form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing continuing review form online
Follow the steps below to use a professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit continuing review form. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out continuing review form
How to fill out a continuing review form:
01
Start by carefully reading the instructions provided on the form. Make sure you understand the purpose of this review and any specific requirements.
02
Gather all the necessary information and documentation required to complete the form. This may include previous study protocols, consent forms, adverse event reports, and any other relevant materials.
03
Begin by providing basic information about the research study, such as the study title, researcher's name, contact information, and any identifying numbers or codes associated with the study.
04
Next, answer any questions regarding the progress of the study. This may include information about recruitment, enrollment numbers, current status of participants, and any changes made to the study design or procedures since the last review.
05
Evaluate the risk and benefit aspects of the research. Some questions on the form may inquire about the potential harm to participants, how risks are being minimized, and how benefits have been demonstrated or can be expected.
06
Describe any adverse events that have occurred since the last review. Include details about the event, its impact on participants, any actions taken, and any changes in the study as a result.
07
Assess the consent process and any changes made to it. Discuss how informed consent is obtained, whether it is ongoing, and any modifications made to the consent form or process.
08
Provide information about any amendments or modifications made to the study protocol since the last review. Explain the reasons for these changes and their potential impact on participant safety or study outcomes.
09
Discuss any data or interim analyses conducted during the study as well as their implications. Share any significant findings, data integrity issues, or changes made as a result of the analyses.
10
Finally, make sure to sign and date the form once you have completed all the necessary sections. Attach any supporting documents or materials as required.
Who needs a continuing review form?
01
Researchers involved in human subjects research need to complete a continuing review form. This form is usually required when conducting studies that involve human participants to ensure that the ongoing research is ethical, safe, and adheres to any regulations or guidelines.
02
Institutional Review Boards (IRBs) or Ethics Committees overseeing the research study also need the continuing review form. They use the information provided in the form to assess the progress of the study, review any changes, and make decisions regarding the ongoing approval of the research.
03
Regulatory bodies or funding organizations may also require the submission of continuing review forms as part of their oversight responsibilities. This helps ensure compliance with regulations, guidelines, and ethical standards.
In summary, a continuing review form must be filled out by researchers involved in human subjects research to assess the progress, safety, and ethical considerations of their study. This information is used by IRBs, ethics committees, and regulatory bodies to make decisions regarding the ongoing approval of the research.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an electronic signature for the continuing review form in Chrome?
You can. With pdfFiller, you get a strong e-signature solution built right into your Chrome browser. Using our addon, you may produce a legally enforceable eSignature by typing, sketching, or photographing it. Choose your preferred method and eSign in minutes.
How do I fill out continuing review form using my mobile device?
You can easily create and fill out legal forms with the help of the pdfFiller mobile app. Complete and sign continuing review form and other documents on your mobile device using the application. Visit pdfFiller’s webpage to learn more about the functionalities of the PDF editor.
Can I edit continuing review form on an iOS device?
Create, modify, and share continuing review form using the pdfFiller iOS app. Easy to install from the Apple Store. You may sign up for a free trial and then purchase a membership.
What is continuing review form?
Continuing review form is a document used to assess the progress of an ongoing research study and ensure that it continues to meet ethical and regulatory standards.
Who is required to file continuing review form?
Researchers and their institutions are required to file a continuing review form for research studies that involve human subjects.
How to fill out continuing review form?
Continuing review forms are typically completed online through the institution's research compliance system. Researchers must provide updates on study progress, any adverse events, and any modifications to the research protocol.
What is the purpose of continuing review form?
The purpose of the continuing review form is to ensure that research studies involving human subjects are being conducted ethically and in accordance with regulations.
What information must be reported on continuing review form?
Researchers must report any changes to the study protocol, adverse events, and progress updates on the continuing review form.
Fill out your continuing review form online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Continuing Review Form is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















