Get the free CENTRAL DRUG RESEARCH INSTITUTE GST No - CSIR-CDRI - cdri res
Show details
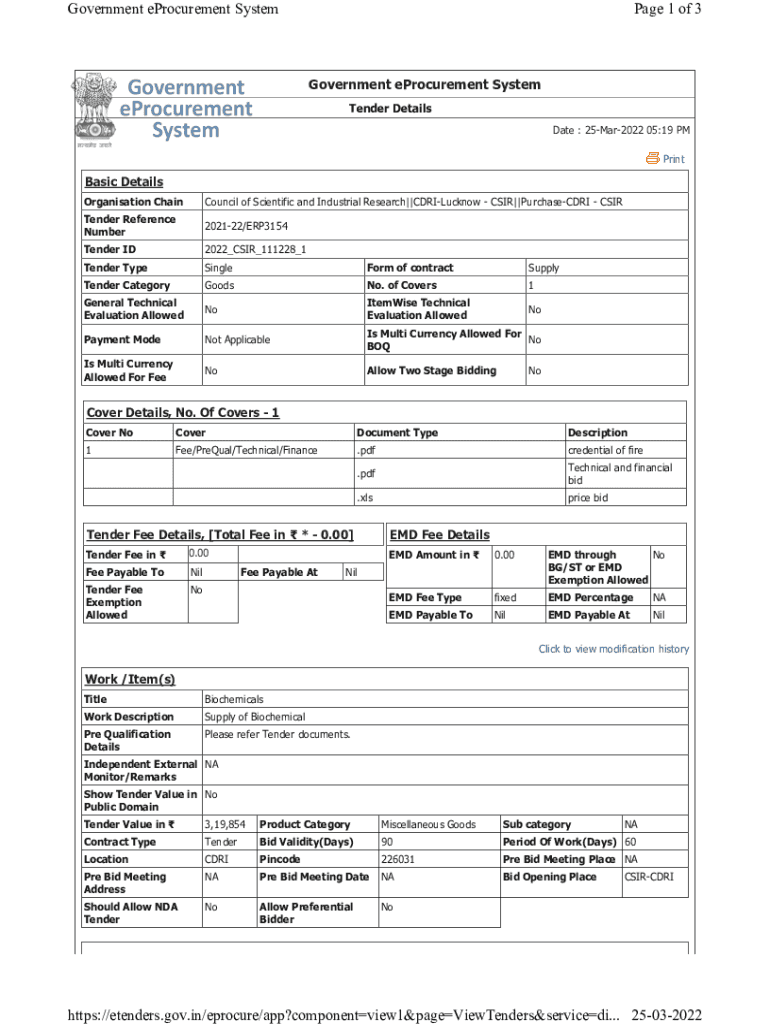
Government procurement System Page 1 of 3Government procurement System Tender Details Date : 25Mar2022 05:19 PM PrintBasic Details Organization ChainCouncil of Scientific and Industrial Research||CDRILucknow
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign central drug research institute

Edit your central drug research institute form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your central drug research institute form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit central drug research institute online
Use the instructions below to start using our professional PDF editor:
1
Log in to your account. Click Start Free Trial and register a profile if you don't have one yet.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit central drug research institute. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out central drug research institute

How to fill out central drug research institute
01
Obtain the application form and guidelines from the official website of Central Drug Research Institute (CDRI).
02
Fill out the application form with accurate and complete information.
03
Attach all required documents such as academic transcripts, certificates, and any other relevant paperwork.
04
Submit the completed application form and documents either online or in person as per the instructions provided.
05
Keep a copy of the application form and documents for your records.
Who needs central drug research institute?
01
Researchers and scientists looking to conduct drug research and develop new pharmaceutical products.
02
Pharmaceutical companies seeking collaboration and expertise in drug discovery and development.
03
Healthcare professionals interested in staying updated on the latest advancements in drug research and therapy.
04
Academic institutions and students pursuing research in the field of pharmaceutical sciences.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send central drug research institute to be eSigned by others?
When you're ready to share your central drug research institute, you can swiftly email it to others and receive the eSigned document back. You may send your PDF through email, fax, text message, or USPS mail, or you can notarize it online. All of this may be done without ever leaving your account.
Can I create an electronic signature for the central drug research institute in Chrome?
Yes. You can use pdfFiller to sign documents and use all of the features of the PDF editor in one place if you add this solution to Chrome. In order to use the extension, you can draw or write an electronic signature. You can also upload a picture of your handwritten signature. There is no need to worry about how long it takes to sign your central drug research institute.
How do I fill out central drug research institute on an Android device?
Complete central drug research institute and other documents on your Android device with the pdfFiller app. The software allows you to modify information, eSign, annotate, and share files. You may view your papers from anywhere with an internet connection.
What is central drug research institute?
Central Drug Research Institute (CDRI) is a premier research organization in India that conducts research and development in the area of drug discovery and development.
Who is required to file central drug research institute?
Pharmaceutical companies and research institutions involved in drug research and development are required to file Central Drug Research Institute reports.
How to fill out central drug research institute?
Central Drug Research Institute reports can be filled out online on the official CDRI website or submitted in person at the institute.
What is the purpose of central drug research institute?
The purpose of Central Drug Research Institute is to track and monitor the progress of drug research and development activities in India, and ensure compliance with regulatory requirements.
What information must be reported on central drug research institute?
Central Drug Research Institute reports must include details of current research projects, funding sources, collaborators, and progress reports on drug development.
Fill out your central drug research institute online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Central Drug Research Institute is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















