
Get the free Current Good Manufacturing Practice and Hazard Analysis ...
Show details
FOOD LAW Act 92 of 2000 AN ACT to codify the licensure and regulation of certain persons engaged in processing, manufacturing, production, packing, preparing, repacking, canning, preserving, freezing,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign current good manufacturing practice
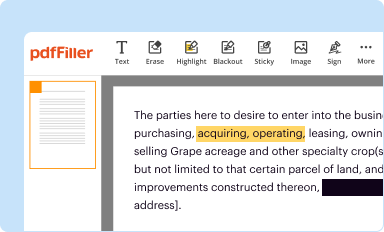
Edit your current good manufacturing practice form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
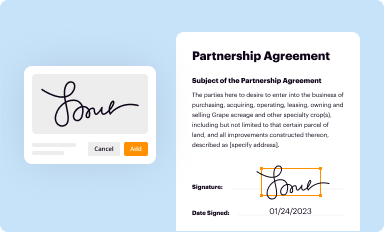
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your current good manufacturing practice form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing current good manufacturing practice online
Follow the steps below to benefit from a competent PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit current good manufacturing practice. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out current good manufacturing practice
How to fill out current good manufacturing practice
01
Understand the requirements of current good manufacturing practice (cGMP) as per regulatory guidelines.
02
Ensure all employees involved in manufacturing processes are trained on cGMP.
03
Establish written procedures for all aspects of manufacturing that impact product quality.
04
Implement adequate control measures to prevent contamination, mix-ups, and errors.
05
Maintain accurate and detailed records of all manufacturing activities.
06
Regularly review and update procedures to stay compliant with cGMP regulations.
Who needs current good manufacturing practice?
01
Pharmaceutical companies
02
Food and beverage manufacturers
03
Cosmetics companies
04
Medical device manufacturers
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my current good manufacturing practice directly from Gmail?
You may use pdfFiller's Gmail add-on to change, fill out, and eSign your current good manufacturing practice as well as other documents directly in your inbox by using the pdfFiller add-on for Gmail. pdfFiller for Gmail may be found on the Google Workspace Marketplace. Use the time you would have spent dealing with your papers and eSignatures for more vital tasks instead.
Can I create an eSignature for the current good manufacturing practice in Gmail?
When you use pdfFiller's add-on for Gmail, you can add or type a signature. You can also draw a signature. pdfFiller lets you eSign your current good manufacturing practice and other documents right from your email. In order to keep signed documents and your own signatures, you need to sign up for an account.
How do I edit current good manufacturing practice on an Android device?
With the pdfFiller Android app, you can edit, sign, and share current good manufacturing practice on your mobile device from any place. All you need is an internet connection to do this. Keep your documents in order from anywhere with the help of the app!
What is current good manufacturing practice?
Current Good Manufacturing Practice (cGMP) is a set of regulations enforced by the FDA to ensure the quality, safety, and efficacy of pharmaceutical products.
Who is required to file current good manufacturing practice?
Manufacturers and distributors of pharmaceutical products are required to file cGMP.
How to fill out current good manufacturing practice?
cGMP must be filled out with detailed information about the manufacturing processes, quality control measures, and documentation procedures.
What is the purpose of current good manufacturing practice?
The purpose of cGMP is to ensure that pharmaceutical products are consistently produced and controlled according to quality standards.
What information must be reported on current good manufacturing practice?
Information such as manufacturing processes, quality control measures, and documentation procedures must be reported on cGMP.
Fill out your current good manufacturing practice online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Current Good Manufacturing Practice is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















