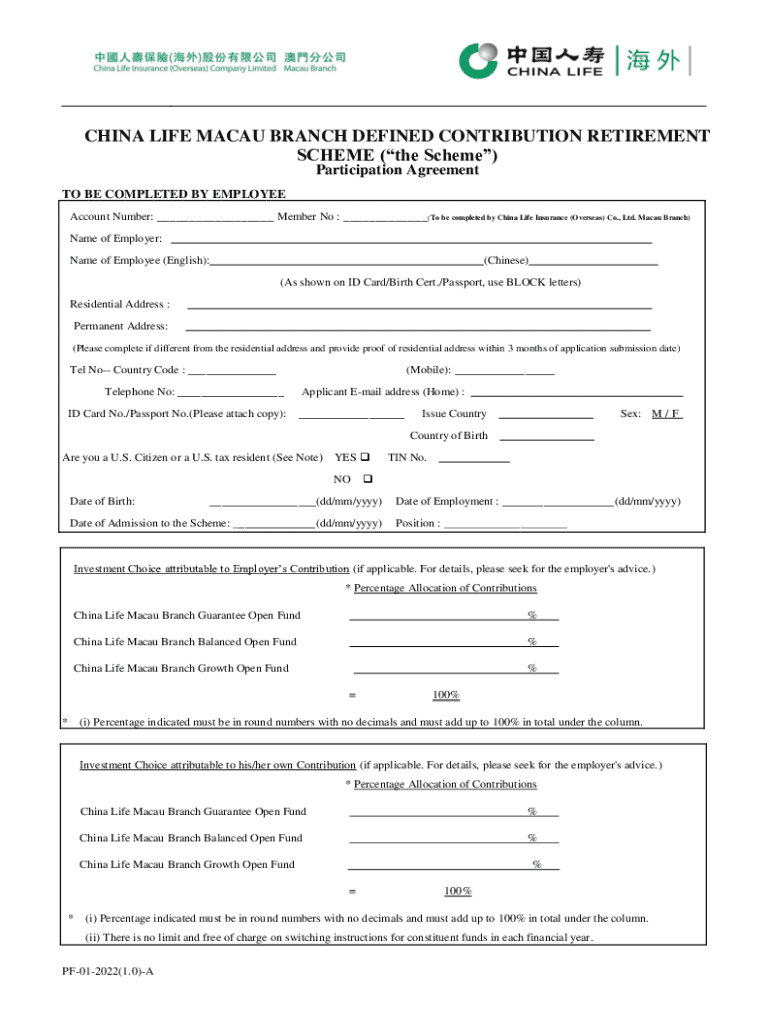
Get the free Cessation of Participation Form
Get, Create, Make and Sign cessation of participation form



How to edit cessation of participation form online
Uncompromising security for your PDF editing and eSignature needs
How to fill out cessation of participation form

How to fill out cessation of participation form
Who needs cessation of participation form?
Cessation of Participation Form: A Comprehensive How-to Guide
Understanding the cessation of participation form
A cessation of participation form is a crucial document that formally declares an individual's decision to withdraw from a study, program, or activity. Whether in healthcare settings like clinical trials, research studies, or corporate initiatives, this form not only communicates the withdrawal but also protects the rights of both participants and organizations involved.
The importance of this form cannot be overstated. For participants, it ensures that their decision is respected and documented, thereby safeguarding their legal and ethical rights. From an organizational standpoint, it helps mitigate the risks associated with unexpected withdrawals, such as compliance issues and the integrity of ongoing assessments.
Scenarios necessitating a cessation of participation form can vary widely. They may include a participant's decision to exit a clinical trial due to adverse effects, an individual's choice to withdraw from a research study because of personal reasons, or even an employee’s removal from a corporate wellness program.
Who needs a cessation of participation form?
Both individuals and organizations need to understand the framework surrounding the cessation of participation form. On the individual side, participants have specific rights and responsibilities to uphold. They can withdraw from programs or studies anytime, yet they must ensure their decision is communicated officially through the cessation of participation form.
Organizations, on the other hand, bear significant legal implications. Failing to acknowledge a withdrawal can lead to ethical dilemmas and may even result in legal repercussions. This is particularly relevant in strict regulatory environments, such as clinical trials governed by the FDA or similar bodies.
Key components of the cessation of participation form
When drafting or filling out a cessation of participation form, it's essential to understand its key components. These sections serve specific functions that contribute to the overall clarity and legality of the document.
It's worth noting that while these components are typically seen across most cessation forms, specific contexts like clinical trials may require additional details or modifications tailored to their regulatory standards.
Step-by-step guide to filling out the cessation of participation form
To ensure a smooth withdrawal process, it’s essential to follow a clear and systematic approach to filling out the cessation of participation form. Here’s a step-by-step guide.
Editing and customizing your cessation of participation form
Customizing your cessation of participation form can enhance its relevance and clarity. Utilizing tools like pdfFiller allows users to effortlessly edit forms to suit their specific needs. From adjusting terms to adding personal notes, customization is key to crafting a document that truly represents the individual’s withdrawal.
Maintaining clarity and professionalism enhances the likelihood that your cessation of participation form will be processed smoothly and without issues.
Managing and submitting your cessation of participation form
Once your cessation of participation form is filled out and customized, the next critical step is managing its submission. Depending on the organization, submission methods can vary: individuals can often submit their forms online, via mail, or in person. Understanding your submission options is crucial for ensuring timely processing of your request.
After submitting the form, tracking its status may be important. Organizations often send confirmation notices, so being aware of what to expect can alleviate anxiety. Also, remember that safeguarding your data and privacy should be a priority during this process.
Implications of cessation of participation
Understanding the implications of cessation of participation is vital for both participants and organizations. For participants, the most immediate effects may include the loss of access to study data or benefits associated with the program they were part of. This can be particularly significant in the case of clinical trials, where ongoing health evaluations are often tied to participation.
Furthermore, participants should be informed about any potential data retention policies that the organization may enforce post-cessation. Many organizations retain participant data for a specified duration, often to comply with regulatory requirements, so awareness of these policies is important.
For organizations, ethical obligations come into play. They are responsible for ensuring that the withdrawal process respects the participant's decision while still complying with regulations. This might include reviewing procedures to tighten the protection of remaining participant data and manage future interactions with withdrawn participants.
Frequently asked questions
As with any administrative process, individuals often have questions surrounding the cessation of participation form. Common issues can arise during the filling-out process, including the appropriate way to convey reasons for withdrawal. Participants might struggle with effectively articulating their experience or the emotional weight of their decision.
Being equipped with answers to these FAQs can significantly ease concerns during an already stressful time, allowing for a smoother transition.
Enhancing your experience with pdfFiller
Utilizing pdfFiller to manage your cessation of participation form allows for a distinctive and enriched experience. The platform boasts features that enable seamless document management — users can edit PDFs, eSign documents, and collaborate in real-time, all from a single, cloud-based platform.
Furthermore, pdfFiller offers a variety of templates for different types of cessation forms, ensuring that users have the right resources at their fingertips to facilitate effective communication during such decisions.
Conclusion: ensuring a smooth transition
Completing a cessation of participation form accurately is essential for a smooth withdrawal process. This guide emphasizes the importance of understanding the context and implications of your decision. By utilizing tools provided by pdfFiller, you can streamline your document management experience, ensuring accuracy and efficiency.
Ultimately, managing your documents effectively not only empowers you but also safeguards your rights and fosters positive relationships with organizations involved in your participation.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send cessation of participation form for eSignature?
How do I edit cessation of participation form in Chrome?
Can I edit cessation of participation form on an Android device?
What is cessation of participation form?
Who is required to file cessation of participation form?
How to fill out cessation of participation form?
What is the purpose of cessation of participation form?
What information must be reported on cessation of participation form?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.















