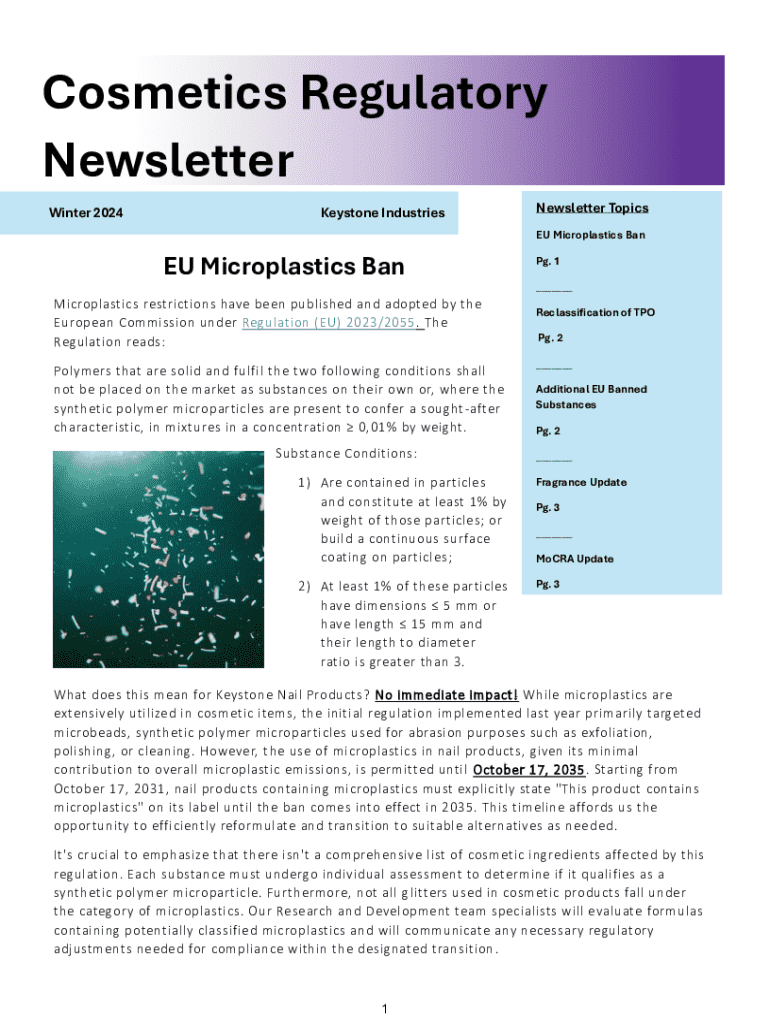
Get the free Cosmetics Regulatory Newsletter Winter 2024
Get, Create, Make and Sign cosmetics regulatory newsletter winter



How to edit cosmetics regulatory newsletter winter online
Uncompromising security for your PDF editing and eSignature needs
How to fill out cosmetics regulatory newsletter winter

How to fill out cosmetics regulatory newsletter winter
Who needs cosmetics regulatory newsletter winter?
Cosmetics Regulatory Newsletter Winter Form: Your Essential Guide
Overview of cosmetics regulatory compliance
Compliance is a critical component in the cosmetics industry, influencing everything from product safety to marketability. Regulatory compliance ensures that products meet the safety, quality, and efficacy standards set forth by governing bodies. Failure to comply can result in severe penalties, recalls, and damage to brand reputation.
Key regulatory bodies include the FDA in the United States, the European Commission in the EU, and other national authorities tasked with overseeing cosmetic safety. These organizations provide guidelines and regulations that industry participants must adhere to ensure consumer safety.
Recent trends are pushing for greater transparency, particularly in ingredient sourcing and disclosure, with a notable increase in scrutiny surrounding claims of 'natural' and 'organic' cosmetics. Moreover, international regulations are evolving, requiring brands to stay agile and well-informed on compliance to navigate these changes effectively.
Winter edition highlights
This winter edition brings noteworthy updates in the cosmetics regulatory landscape. Among these, there is a heightened focus on natural and organic products, emphasizing sustainability and ethical sourcing. Brands are encouraged to be proactive in reformulating products that align with these new guidelines.
Additionally, major changes are underway regarding ingredient safety assessments. Regulatory authorities are emphasizing the need for detailed safety profiles on both traditional and novel ingredients, impacting formulation strategies and compliance protocols.
Winter form: Step-by-step guide
The winter form is an essential document for brands aiming to maintain compliance with evolving cosmetics regulations. Understanding its purpose and functionality is critical for successful submissions.
Completing the winter form involves several steps, starting with gathering essential information such as product details, ingredient declarations, and safety assessments.
Visual aids in the form of annotated examples of filled-out forms can serve as an invaluable resource. They simplify the process by illustrating what a completed winter form should look like, thus alleviating uncertainties during completion.
Tips for successful submission
Understanding the timeline for submission and review is key to successful compliance. Brands should be aware that regulatory bodies often operate on specific timelines, so submitting documents promptly will allow for sufficient review time.
Navigating common compliance challenges is inevitable; however, proactive communication with regulatory authorities can often clarify uncertainties and expedite the review process.
Regulatory insights from recent newsletters
The latest winter newsletter summarizes critical articles that shed light on the evolving regulatory landscape. Key topics include insights on new ingredient regulations and how they affect compliance strategies for brands across different markets.
Expert opinions within the newsletter provide additional context regarding upcoming regulatory changes and their anticipated impact on the industry. Case studies from leading brands illustrate successful navigation of these regulations, offering practical insights for others.
Tools and resources
pdfFiller offers interactive tools for document management tailored for the cosmetics industry. Features like eSignatures streamline the signing process, facilitating quick approvals while providing legal compliance.
Accessing previous newsletters and relevant regulatory publications via pdfFiller can keep users informed on past compliance standards and evolving best practices.
Community engagement
Engagement with industry professionals fosters shared insights and enhanced learning. Encouraging feedback on this winter edition's contents can lead to improved future renditions.
Opportunities for networking and collaboration within webinars and workshops focused on regulatory changes create an invaluable community support system.
FAQs about cosmetics regulation
As the regulatory landscape evolves, common questions arise regarding procedures and terminology. It is essential for professionals to seek clarity on these aspects to navigate compliance seamlessly.
Expert answers from regulatory practitioners can help clarify complex queries, ensuring that cosmetics brands remain compliant with the latest standards and practices.
Glossary of terms
A well-defined glossary of essential terminology is paramount for understanding compliance documents. Key acronyms and terminologies surrounding cosmetics regulation enhance clarity and communication among stakeholders.
Contact and subscription information
To stay informed about future editions of the cosmetics regulatory newsletter, subscribing is crucial. Users can register easily via the pdfFiller platform to receive timely updates.
For any inquiries related to regulations, users can access contact information directly within pdfFiller for swift support.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in cosmetics regulatory newsletter winter?
Can I create an eSignature for the cosmetics regulatory newsletter winter in Gmail?
How do I edit cosmetics regulatory newsletter winter on an Android device?
What is cosmetics regulatory newsletter winter?
Who is required to file cosmetics regulatory newsletter winter?
How to fill out cosmetics regulatory newsletter winter?
What is the purpose of cosmetics regulatory newsletter winter?
What information must be reported on cosmetics regulatory newsletter winter?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.


















