Get the free protocol tracking sheet
Get, Create, Make and Sign protocol tracking sheet

Editing protocol tracking sheet online
Uncompromising security for your PDF editing and eSignature needs
How to fill out protocol tracking sheet
How to fill out protocol tracking sheet
Who needs protocol tracking sheet?
Protocol Tracking Sheet Form: A Comprehensive Guide
Understanding the protocol tracking sheet form
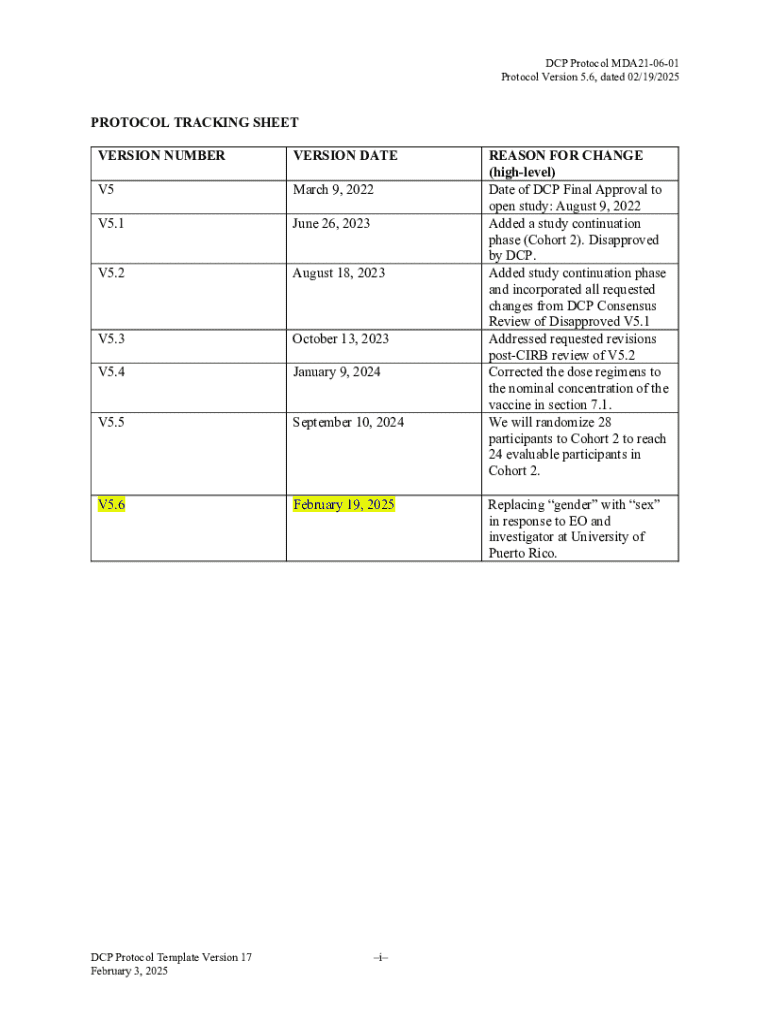
A protocol tracking sheet is a vital document used primarily in research and project management to maintain an organized record of various protocols being followed. Essentially, it serves to monitor the progress of different tasks involved in a research study or project, ensuring that all procedural guidelines are adhered to. This document is indispensable for both compliance and communication among team members involved in a particular study.
The importance of a protocol tracking sheet cannot be underestimated. It not only aids in tracking the status of ongoing projects but also boasts a historical record that can be invaluable during audits, reviews, or future project planning. When accurately completed, it contributes to more efficient workflow management and promotes accountability within teams.
Key components of a protocol tracking sheet
A well-structured protocol tracking sheet comprises several key components that facilitate its primary function. Commonly included sections are the protocol title, version control, associated documents, study description, timeline, and compliance signatures. Each of these sections plays a crucial role in ensuring that the document serves its purpose effectively.
For instance, the protocol title provides a clear identification of the study, while version control helps keep track of edits and updates over time. The study description section articulates the primary objectives and hypotheses, which are vital for the team’s focus. Lastly, including a timeline assists in project management by specifying deadlines and progress checkpoints.
Preparing to fill out the protocol tracking sheet
Before diving into filling out your protocol tracking sheet, it’s imperative to identify its purpose clearly. Understanding why you are creating this document will guide you in determining the specific requirements necessary for your project. For example, a clinical trial may necessitate more stringent documentation compared to initial exploratory research.
To ensure your protocol tracking sheet is comprehensive and effective, start by gathering all necessary information. This typically includes project details such as study specifics, participating team members, and relevant timelines. Each piece of data contributes to crafting a holistic and functional document.
Utilizing collaborative tools such as pdfFiller can streamline this process, allowing teams to work together in compiling necessary information effectively.
Step-by-step instructions for filling out the protocol tracking sheet
Creating a protocol tracking sheet begins with initiating the form on the pdfFiller platform. Here's a straightforward step-by-step guide to making your task easier.
Start by navigating to the pdfFiller platform and selecting the appropriate template that aligns with your needs. From there, initiate a new document. Make sure it adheres to the guidelines and layout requirements of the protocol you are handling.
Detailed walkthrough of each section
1. **Basic Information:** Input the protocol title along with the lead investigator's name. Don’t forget the project start and end dates to keep track of critical timelines.
2. **Version Control:** This section is crucial for documenting any changes made to the protocol. Use a clear numbering and date format to ensure every version is easily identifiable.
3. **Study Description:** Articulate the study's goals, objectives, and any hypotheses here. This section informs team members and stakeholders about the project’s essence.
4. **Timeline:** Develop an effective timeline by identifying milestones and deadlines for key tasks. This visual line of progression can significantly aid workflow.
5. **Compliance and Signatures:** Ensure compliance checks are noted, and gather necessary signatures to endorse the protocol officially.
Utilizing pdfFiller’s editing tools will enhance your experience, allowing you to incorporate text boxes, annotations, and enable real-time collaboration among your team.
Editing and customizing your protocol tracking sheet
Editing and customizing your protocol tracking sheet is essential for optimizing its performance and relevance to the project at hand. pdfFiller offers a range of tools that make this process straightforward and efficient.
Utilize the editing suite to adjust font sizes, layout, and even color coding elements to draw attention to critical areas. This level of customization ensures that the document remains user-friendly and visually organized.
When personalizing your document, consider which specific components are most relevant to your team and project. This may include unique sections for comments, external reviews, or additional tracking elements tailored to your research needs.
Managing and sharing your protocol tracking sheet
Once your protocol tracking sheet is filled out and customized, it's time to save and store the document. pdfFiller allows you a variety of file format options, with PDF being the most compatible for compliance and sharing with stakeholders.
To ensure broader accessibility and collaboration, pdfFiller also provides methods to share the tracking sheet with team members. You can invite others via email or share links that allow them to view or edit the document, enhancing collective input and oversight.
Version management is a powerful feature in pdfFiller. As your protocol tracking sheet undergoes modifications, keeping track of different versions helps avoid confusion and maintains a clear history of changes, which is essential for audits.
Common challenges and troubleshooting
Utilizing a protocol tracking sheet can present unique challenges, ranging from writer's block to difficulties in collating comprehensive data. These are common issues many face when tasked with documentation.
To combat these obstacles, consider setting aside dedicated times specifically for documenting progress, encouraging a focused approach. Additionally, build a habit of collecting data consistently to avoid information gaps, ensuring that whenever a document needs updating, all relevant information is readily available.
Frequently asked questions can help clarify common queries about the protocol tracking form’s usage. It's beneficial to compile these and refer to them frequently to enhance familiarization with the document's structure and purpose.
Case studies and examples
Real-world applications of protocol tracking sheets can be found across various fields such as clinical research, corporate projects, and educational studies. These cases reveal how effectively constructed tracking sheets enhance project management.
For instance, in clinical trials, a protocol tracking sheet serves as a backbone that offers regulatory compliance and outlines the progression of different study phases. Successful implementations have proven that clear documentation can streamline communication, reduce errors, and promote accountability among teams.
The lessons learned from these implementations highlight the necessity for protocol tracking sheets to be routinely optimized to meet evolving needs within their respective fields.
Additional interactive tools on pdfFiller
In addition to the protocol tracking sheet, pdfFiller offers various document management resources that cater to a wide array of documentation needs. Templates related to protocols, project tracking, and real-time collaboration features can enhance your overall workflow.
Moreover, interactive tutorials on pdfFiller provide users with step-by-step guidance, enhancing the learning experience and empowering them to utilize the platform fully. Accessing these resources can significantly enhance one’s efficiency in document creation and management.
Conclusion of key insights on protocol tracking sheet form
The protocol tracking sheet form is instrumental in ensuring effective documentation and collaboration within teams. By meticulously utilizing this resource, organizations can ensure compliance, enhance communication, and streamline project management across various applications.
In conclusion, mastering the art of filling out and managing a protocol tracking sheet can lead to more productive research endeavors and successful project outcomes. pdfFiller empowers users to engage efficiently with their documentation processes, bringing valuable time-saving and organizational benefits.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify protocol tracking sheet without leaving Google Drive?
How do I edit protocol tracking sheet straight from my smartphone?
How do I edit protocol tracking sheet on an iOS device?
What is protocol tracking sheet?
Who is required to file protocol tracking sheet?
How to fill out protocol tracking sheet?
What is the purpose of protocol tracking sheet?
What information must be reported on protocol tracking sheet?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















