Electron Configuration And The Periodic Table
What is Electron Configuration And The Periodic Table?
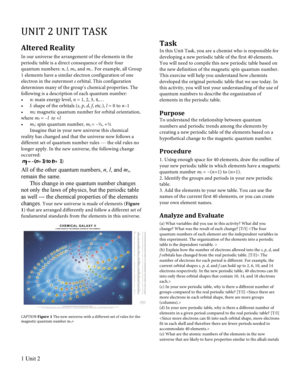
Electron configuration refers to the distribution of electrons in the energy levels or orbitals of an atom. It is a vital concept in chemistry as it helps in understanding the properties and behavior of elements. The periodic table, on the other hand, organizes elements based on their atomic number, electron configuration, and recurring chemical properties. It is a tabular arrangement of all known elements that provides a systematic framework for studying and predicting their characteristics.
What are the types of Electron Configuration And The Periodic Table?
There are different types of electron configurations and periodic tables based on their purposes and variations. Some of the commonly used types include:
How to complete Electron Configuration And The Periodic Table
Completing electron configuration and understanding the periodic table involves a few steps:
pdfFiller empowers users to create, edit, and share documents online. With unlimited fillable templates and powerful editing tools, it is the go-to PDF editor for all your document needs.