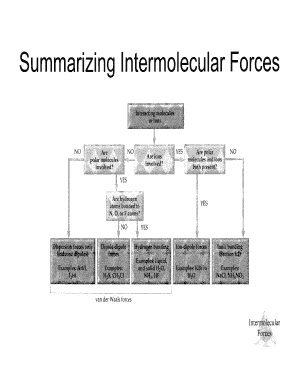
Electronegativity Of Cl
Video Tutorial How to Fill Out electronegativity of cl
Thousands of positive reviews can’t be wrong
Read more or give pdfFiller a try to experience the benefits for yourself
Questions & answers
What is the bond of Cl and C?
Answer and Explanation: The bond that is formed between carbon and chlorine is a type of polar bond. This is because there exists a difference in the values of electronegativity between carbon as well as chlorine.
What is the electronegativity between Cl and Cl?
According to scale (Pauling's) of electronegativity, the chlorine atom has been assigned an electronegativity value of 3.16. As the bond is formed between two Cl atoms thus, the electronegativity difference will be zero (3.16 - 3.16 = 0). Answer: The electronegativity difference for the Cl-Cl bond is 0.
How do you calculate electronegativity?
Note the electronegativity of the first and second elements. How to find electronegativity? Just use a periodic table which includes it and read it for the selected element. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms.
What is the electronegativity of Cl?
chlorine which has a very high electronegativity because it has seven electrons in its valence orbital and needs one more to become stable like a noble gas.
What is the electronegativity of Cl?
As the electronegativity difference between C and Cl is <1.8, therefore, the C-Cl bond is covalent.
How do you calculate the electronegativity of a bond?
One way of estimating the ionic character of a bond—that is, the magnitude of the charge separation in a polar covalent bond—is to calculate the difference in electronegativity between the two atoms: Δχ = χB − χA.
Related templates