Electronegativity Of H
Video Tutorial How to Fill Out electronegativity of h
Thousands of positive reviews can’t be wrong
Read more or give pdfFiller a try to experience the benefits for yourself
Questions & answers
Is H or Si more electronegative?
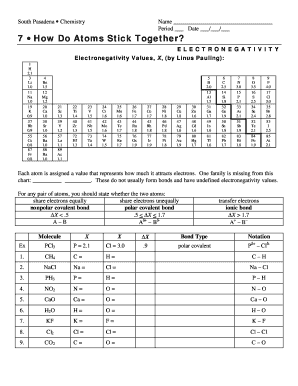
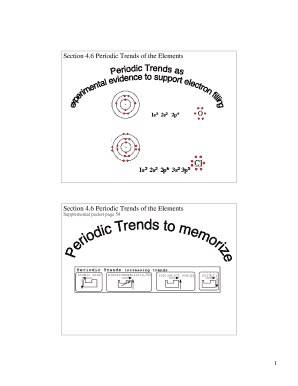
Periodic Trends — Electronegativity 1A4A(1)(14)1H 2.202Li 0.98C 2.553Na 0.93Si 1.909 more rows
How do we calculate electronegativity?
EN(X)−EN(Y)=0.102√Δ. In that equation, the factor 0.102 is simply a conversion factor between kJ and eV to keep the units consistent with bond energies. By assigning a value of 4.0 to Fluorine (the most electronegative element), Pauling was able to set up relative values for all of the elements.
What is the electronegativity difference of Si and H?
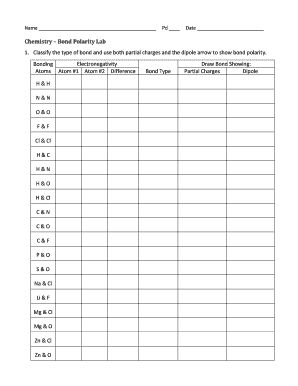
Electronegativity and Bond Type BondElectronegativity DifferencePolarityC–C0.0nonpolarC–H0.4δ−C−δ+HSi–C0.7δ+Si−δ−CSi–O1.7δ+Si−δ−O 27 Oct 2020
Which is the element with the lowest electronegativity?
Hence the electronegativity of cesium is the lowest. The electronegativity of cesium is 0.659.
What is en difference of Si and H?
The difference in electronegativity for both bonds is approximately 0.3, but the C-H bond is considered to be nonpolar covalent, while the Si-H bond is considered to be polar covalent. Because silicon is a larger atom than carbon, it will also have a larger electron cloud.
Which is the correct order of electronegativity F and C?
Hence fluorine is most electronegative and carbon is least electronegative.
Related templates