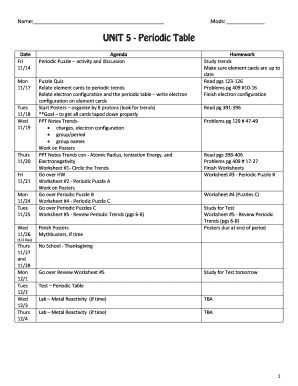
Periodic Table Electronegativity Values
What is periodic table electronegativity values?
Periodic table electronegativity values refer to a numerical scale that measures the ability of atoms to attract electrons towards themselves in a chemical bond. This concept is crucial in understanding the behavior of different elements and predicting how they interact with each other.
What are the types of periodic table electronegativity values?
There are two widely used types of electronegativity values in the periodic table:
Pauling Electronegativity: Named after Linus Pauling, this scale assigns electronegativity values ranging from 0.7 (for elements with the lowest electronegativity) to 4.0 (for elements with the highest electronegativity).
Mulliken Electronegativity: Developed by Robert Mulliken, this scale is based on the average of ionization energy and electron affinity. It provides a different perspective on electronegativity values compared to the Pauling scale.
How to complete periodic table electronegativity values
Completing the periodic table electronegativity values requires a thorough understanding of the elements and their properties. Here are the steps to undertake:
01
Familiarize yourself with the periodic table and the electronegativity values already assigned to different elements.
02
Research and gather reliable data on elements with missing or incomplete electronegativity values.
03
Compare the electronegativity values of similar elements and identify trends or patterns.
04
Use established calculation methods or models to estimate or determine the missing electronegativity values.
05
Double-check the accuracy of the completed values, ensuring they align with the principles of electronegativity and known chemical behavior.
By following these steps, you can contribute to the completion and accuracy of the periodic table electronegativity values, helping scientists and researchers in various fields.
Video Tutorial How to Fill Out periodic table electronegativity values
Thousands of positive reviews can’t be wrong
Read more or give pdfFiller a try to experience the benefits for yourself
Questions & answers
How do you write electronegativity?
1:44 6:30 How to calculate Electronegativity? Easy Trick - YouTube YouTube Start of suggested clip End of suggested clip There are three circles i write 3 then i decrease it by 0.5. I write 2.5. And i decrease it by 0.4 iMoreThere are three circles i write 3 then i decrease it by 0.5. I write 2.5. And i decrease it by 0.4 i write to 0.1. So this is the electronegativity series in the periodic.
How are the elements of electronegativity arranged in order?
Electronegativity increases left to right across a row in the periodic table e.g. C < N < O < F. Electronegativity decreases as you move down a group in the periodic table e.g. F > Cl > Br > I. F is the most electronegative element.
How do you arrange elements in order of decreasing electronegativity?
Electronegativity increases left to right across a row in the periodic table e.g. C < N < O < F. Electronegativity decreases as you move down a group in the periodic table e.g. F > Cl > Br > I. F is the most electronegative element.
What is the order of increasing electronegativity?
The electronegativity increases along the period. It also decreases along the group. Thus considering theses 2 trends, the correct order of electronegativity is N<O<F.
How is electronegativity written?
Electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus.
What are the top 4 electronegative elements?
Again, that's FONCLBRISCH. This is the most electronegative elements on the periodic table starting with the most electronegative on the top, and decreasing in electronegativity as we work down. So we have fluorine, oxygen, nitrogen, chlorine, bromine, iodine, sulfur, carbon, and hydrogen.
Related templates