Consent Sign Request For Free
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent

Upload your document to the PDF editor

Type anywhere or sign your form

Print, email, fax, or export

Try it right now! Edit pdf
Users trust to manage documents on pdfFiller platform
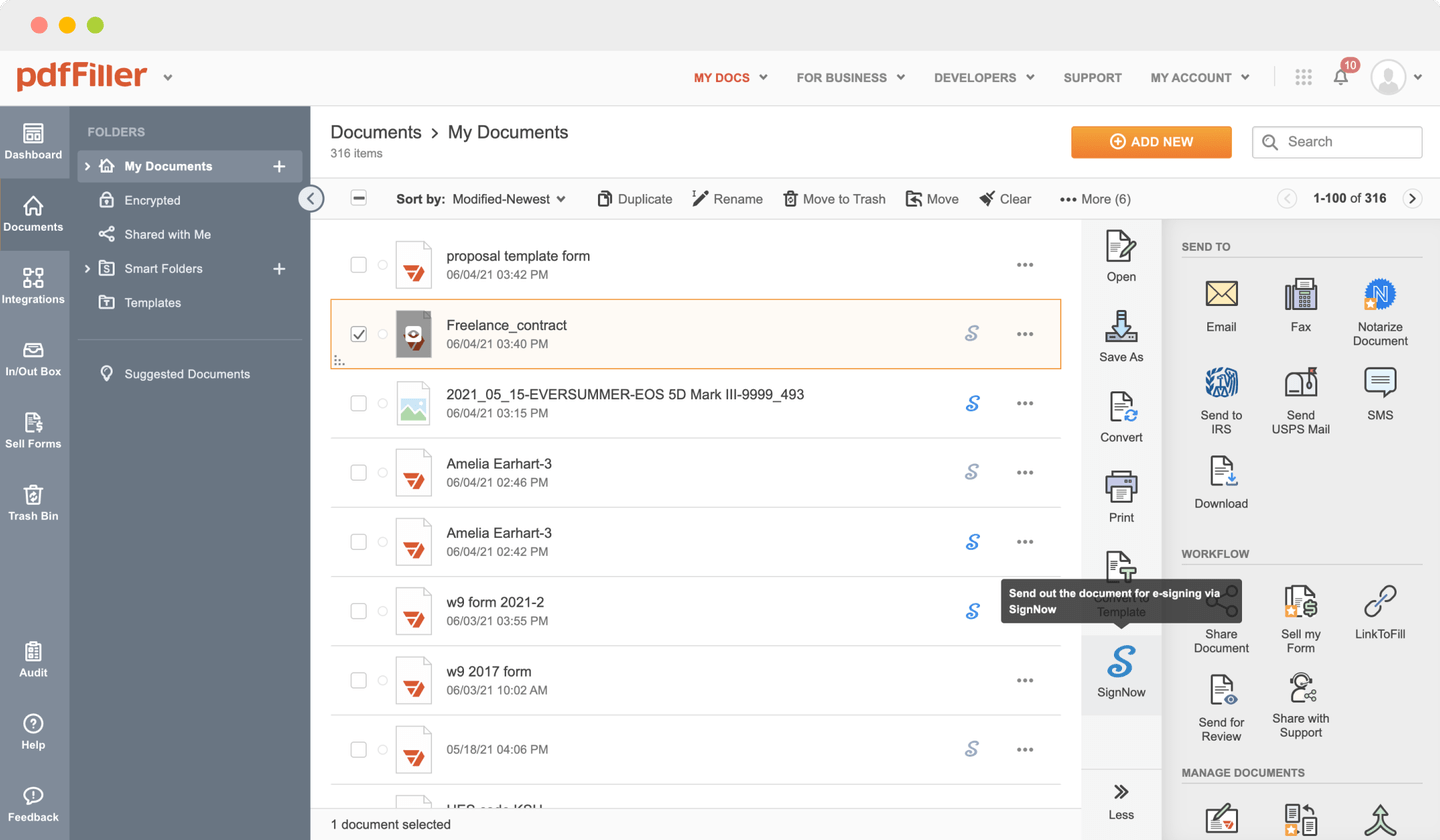
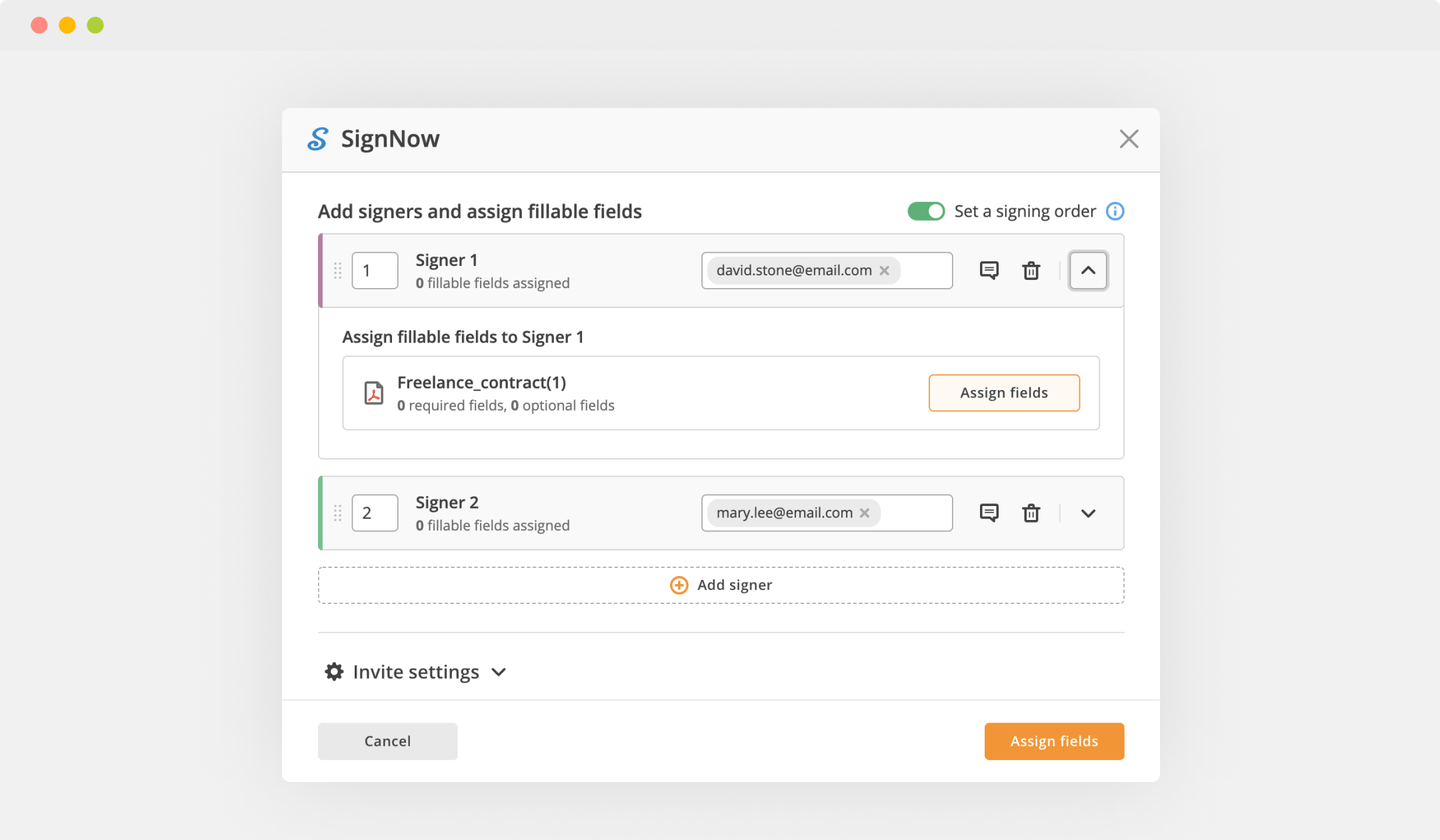
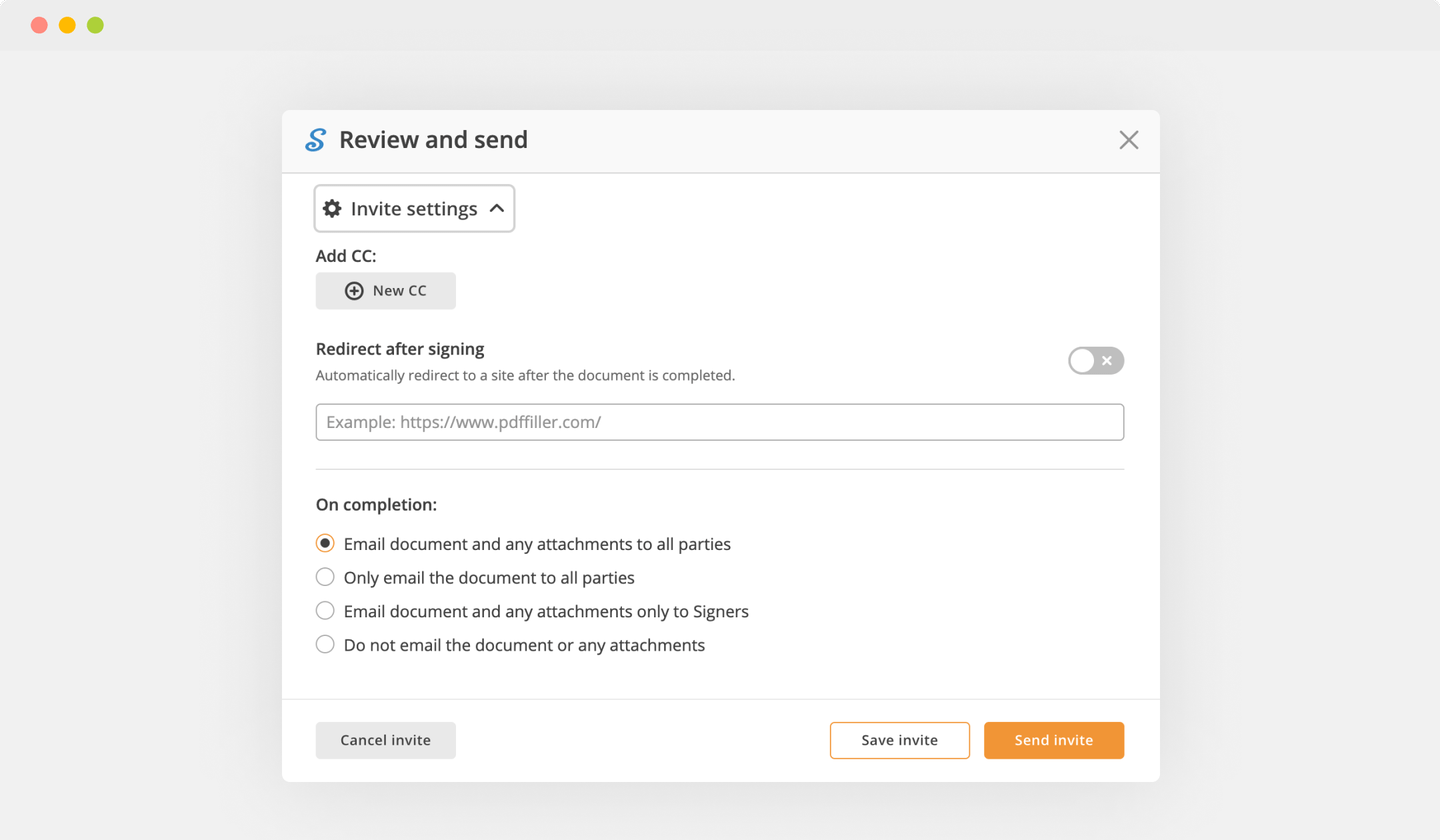
Send documents for eSignature with signNow
Create role-based eSignature workflows without leaving your pdfFiller account — no need to install additional software. Edit your PDF and collect legally-binding signatures anytime and anywhere with signNow’s fully-integrated eSignature solution.
All-in-one PDF software
A single pill for all your PDF headaches. Edit, fill out, eSign, and share – on any device.
pdfFiller scores top ratings in multiple categories on G2
How to Consent Sign Request
Stuck with numerous applications to create and edit documents? Use our solution instead. Use our editor to make the process efficient. Create forms, contracts, make templates and many more features, within your browser. You can Consent Sign Request directly, all features are available instantly. Pay as for a basic app, get the features as of a pro document management tools.
How-to Guide
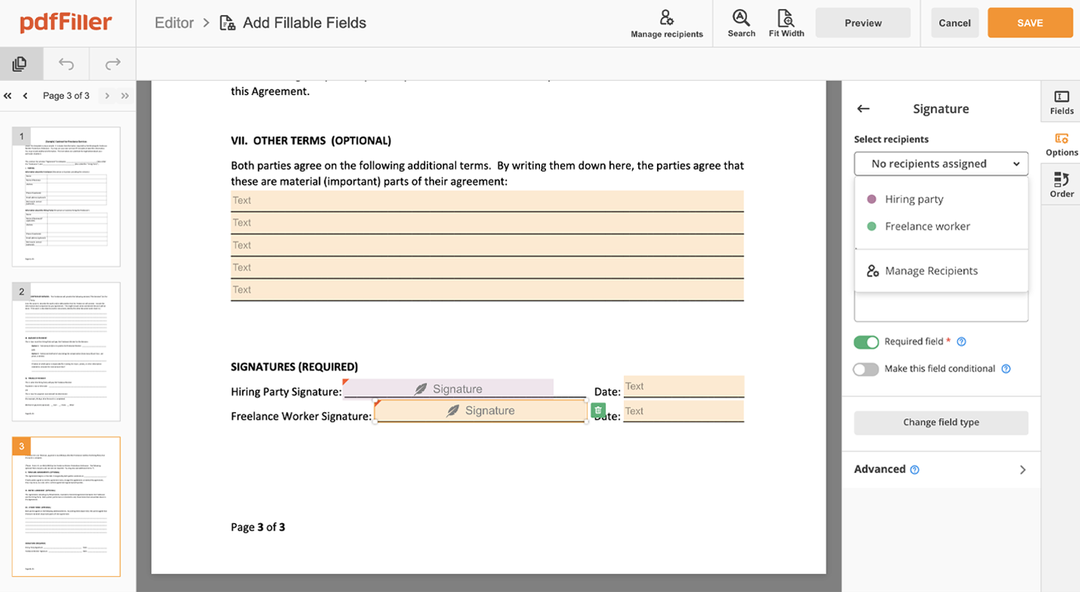
How to edit a PDF document using the pdfFiller editor:
01
Drag and drop your document to pdfFiller`s uploader
02
Select the Consent Sign Request feature in the editor's menu
03
Make all the necessary edits to your file
04
Push the “Done" button in the top right corner
05
Rename the form if needed
06
Print, save or email the form to your device
Video Review on How to Consent Sign Request
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
Dr R
2015-12-27
I was shocked to learn AFTER I PAID that the "monthly" payment touted in 3 big choice boxes was ONLY and effective cost IF I paid an annual fee IN FULL. THAT WAS NOT CLEAR and I feel duped. However, I am extremely pleased with what you offer AND the ease of using your site. It is well worth the 29.99/month vs what I thought was 9.99. You really need to make that clear for your customers, though. Don't start a customer relationship with the customer feeling duped or tricked.

Mike F.
2018-03-26
Easy to use
I couldn't do my job without this tool. I utilized this everyday as I create and fill out forms quite often. Its very simple and does not take any time to understand how to utilize the software.
At times it can give you problems when you try and save and add certain things. I've also had problems copy certain areas.


Get a powerful PDF editor for your Mac or Windows PC
Install the desktop app to quickly edit PDFs, create fillable forms, and securely store your documents in the cloud.

Edit and manage PDFs from anywhere using your iOS or Android device
Install our mobile app and edit PDFs using an award-winning toolkit wherever you go.

Get a PDF editor in your Google Chrome browser
Install the pdfFiller extension for Google Chrome to fill out and edit PDFs straight from search results.
List of extra features
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do you get informed consent?
The informed consent document should succinctly describe the research as it has been presented in the IRB application. Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I). Include a statement of agreement at the conclusion of the informed consent document.
Is informed consent possible?
Informed consent is not possible if the doctor or researcher involved is not a moral character, or is lacking sufficient knowledge of the process under question. Informed consent is not possible if the beliefs in place are not rational, so that reasoning and reaching an informed decision are thus impaired.
What is informed consent and how is it obtained?
When conducting clinical research, the obtaining of informed consent is required. Informed consent is a procedure through which a competent subject, after having received and understood all the research-related information, can voluntarily provide his or her willingness to participate in a clinical trial.
What is an informed consent form and why is it important?
The main purpose of the informed consent process is to protect the patient. A consent form is a legal document that ensures an ongoing communication process between you and your health care provider. Your health care provider works with you to figure out the best way to give you the information you need.
Who signs informed consent?
Important Note: The California Medical Experiment Act requires attestation that the consent form is signed and dated by a person other than the participant or the participant's guardian or legally-authorized representative who can attest that the requirements for informed consent has been met.
Does a physician have to sign an informed consent?
True informed consent is a process of managing a patient's expectations; it is not just a signature on a document. The physician must then provide sufficient information to the patient so that a reasonable and informed decision regarding a treatment plan can be made. This physician responsibility cannot be delegated.
What constitutes informed consent?
Medical Definition of Informed consent: The process by which a patient learns about and understands the purpose, benefits, and potential risks of a medical or surgical intervention, including clinical trials, and then agrees to receive the treatment or participate in the trial.
What are informed consent guidelines?
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations (45 CFR 46.116) provide the framework for the type of information (i.e., the “elements") that must be included as part of the consent process.
What is informed consent and why is it important?
Informed consent creates trust between doctor and patient by ensuring good understanding. It also reduces the risk for both patient and doctor. With excellent communication about risks and options, patients can make choices which are best for them and physicians face less risk of legal action.
How do I make a consent form?
Writing a Consent Form A Consent Form is read by the participant, signed and handed back to the researcher and should include the following features: Use University of Wollongong/AHS letterhead. 1. Provide the title of the research project, the researcher(s) name, supervisor's name (for 2.
What should be included in a consent form?
A statement that the study involves research, an explanation of the purposes of the research, the expected duration of a subject's participation, a description of the procedures to be followed, and if applicable identification of any experimental procedures.
What is a participant consent form?
A consent form is not simply about a person giving you permission to involve them in research, it is an agreement between the researcher and the research participant outlining the roles and responsibilities they are taking towards one another throughout the whole of the research process.
What should be included in an informed consent form?
Valid informed consent for research must include three major elements: (1) disclosure of information, (2) competency of the patient (or surrogate) to make a decision, and (3) voluntary nature of the decision. US federal regulations require a full, detailed explanation of the study and its potential risks.
How do you create a consent form in psychology?
Leave space for the participant to write the date.
It is a written form that participants need to sign.
Be clear if the participants need to be available at a later point.
Must give an outline of what the study involves.
Include space for the participant to write their name in print.
What is a consent form in psychology?
A consent form is not simply about a person giving you permission to involve them in research, it is an agreement between the researcher and the research participant outlining the roles and responsibilities they are taking towards one another throughout the whole of the research process.
eSignature workflows made easy
Sign, send for signature, and track documents in real-time with signNow.