Consent Signatory Request For Free
Upload your document
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent

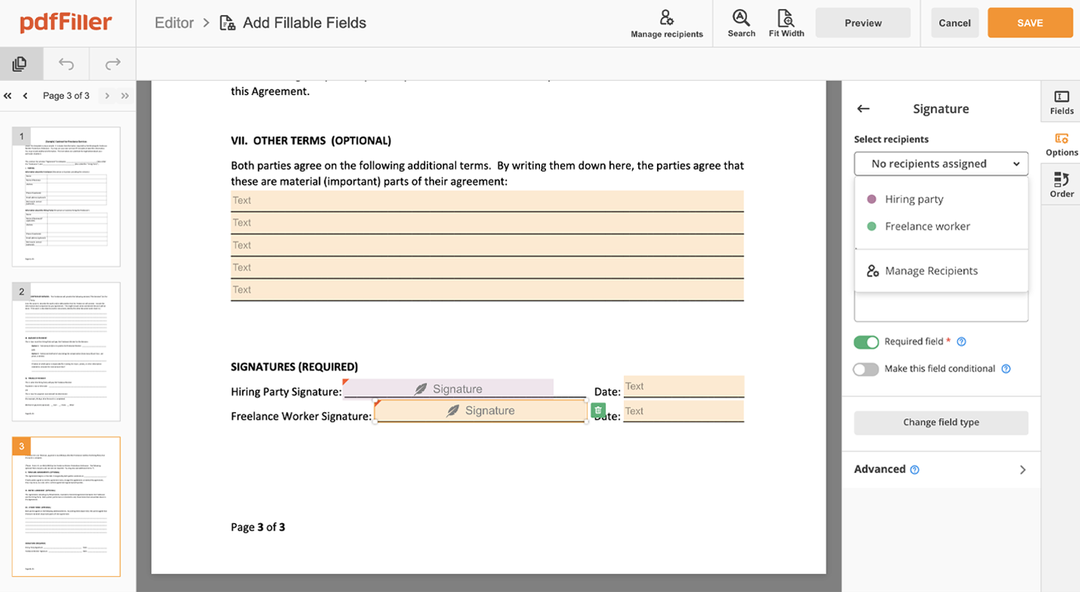
Upload your document to the PDF editor

Type anywhere or sign your form

Print, email, fax, or export

Try it right now! Edit pdf
Users trust to manage documents on pdfFiller platform
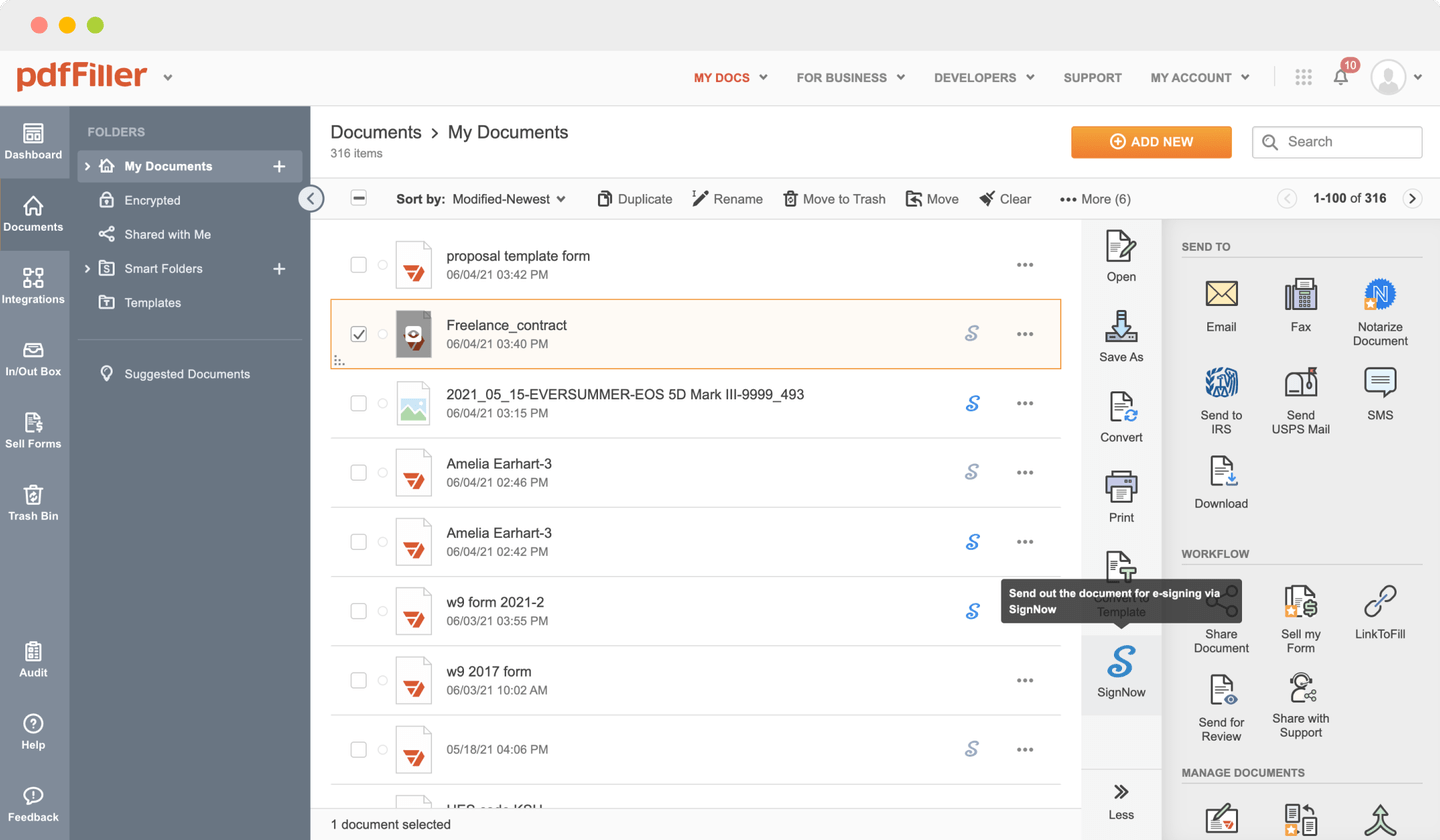
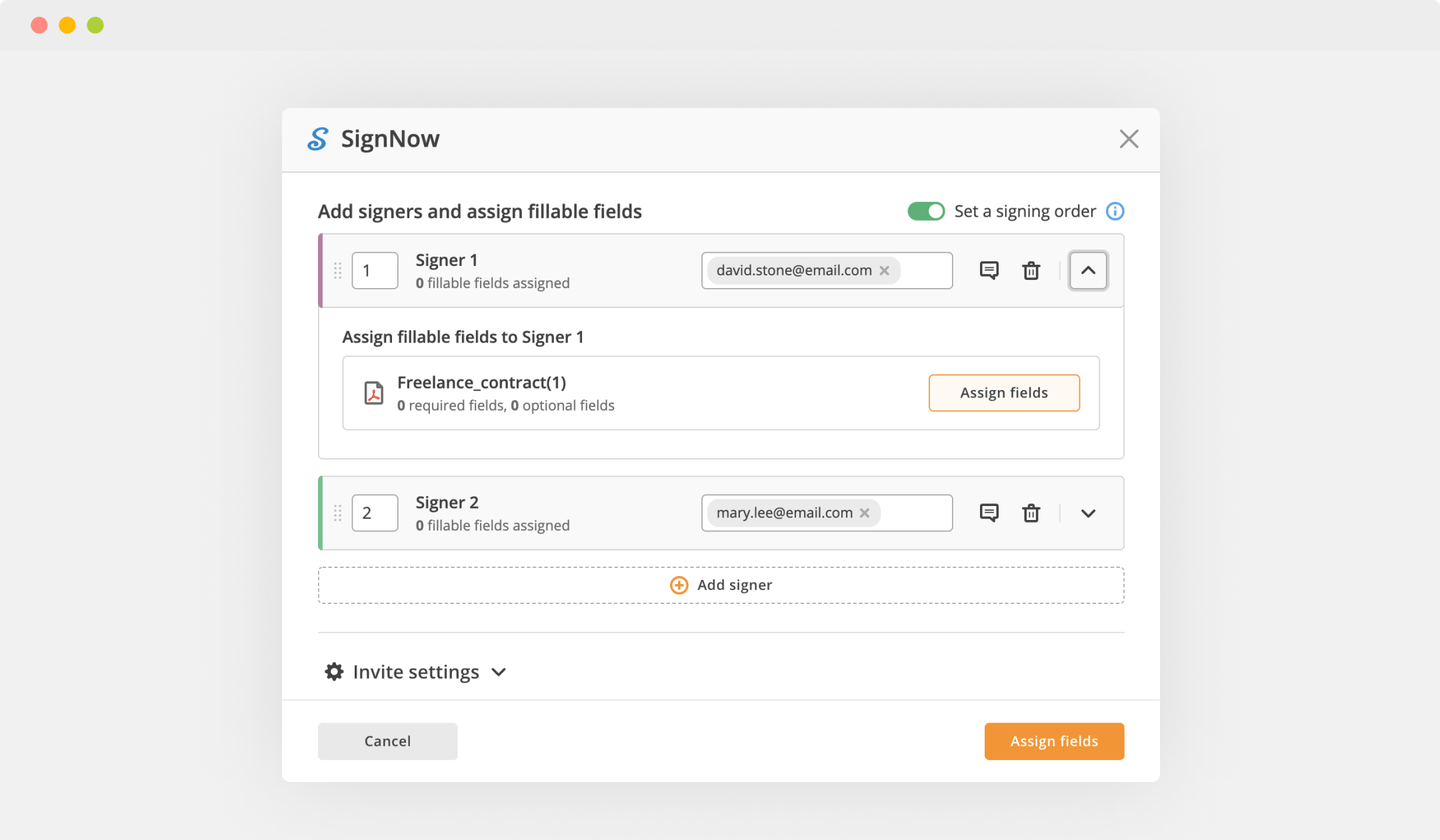
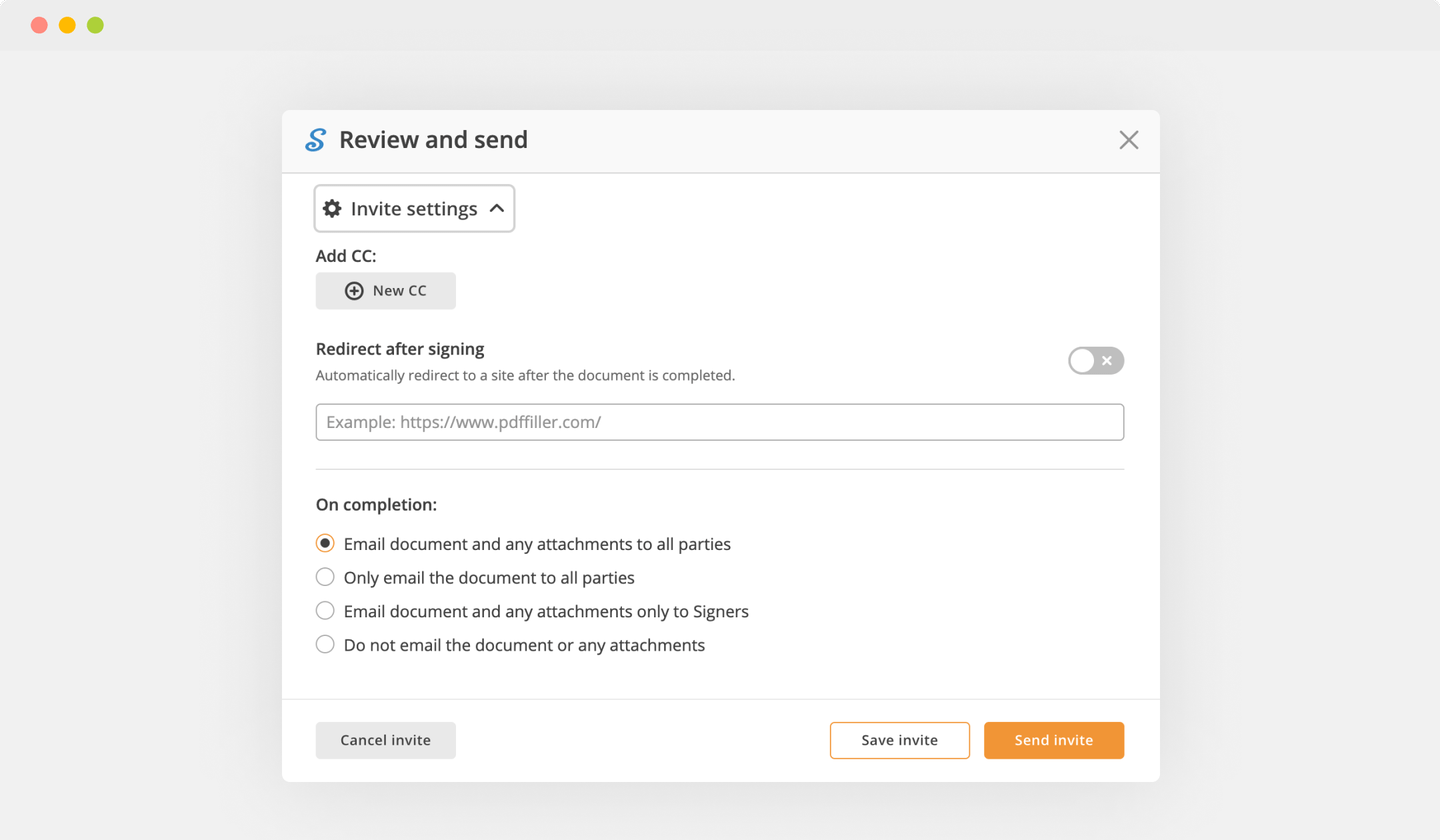
Send documents for eSignature with signNow
Create role-based eSignature workflows without leaving your pdfFiller account — no need to install additional software. Edit your PDF and collect legally-binding signatures anytime and anywhere with signNow’s fully-integrated eSignature solution.
All-in-one PDF software
A single pill for all your PDF headaches. Edit, fill out, eSign, and share – on any device.
pdfFiller scores top ratings in multiple categories on G2
How to Consent Signatory Request
Are you stuck with different applications for editing and signing documents? Use our solution instead. Use our editor to make the process fast and simple. Create forms, contracts, make document templates, integrate cloud services and many more features within one browser tab. You can Consent Signatory Request right away, all features are available instantly. Get an advantage over those using any other free or paid applications.
How-to Guide
How to edit a PDF document using the pdfFiller editor:
01
Upload your template using pdfFiller
02
Find and choose the Consent Signatory Request feature in the editor's menu
03
Make the necessary edits to the document
04
Click the “Done" button at the top right corner
05
Rename your template if it's necessary
06
Print, save or email the document to your desktop
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
Sophie
2016-06-24
Customer service is fantastic, as a result, I will continue using pdf filler and liaising with customer service to improve my experience. I love that they get back to you within 12 hours and that they actually respond to you personally. O did not expect this at all.

User in Banking
2016-08-30
What do you like best?
All-in-one tool that ultimately expedites the process of filling out and scanning forms.
What do you dislike?
Nothing in particular; fixed one-time pricing would be preferable over subscriptions though.
What problems are you solving with the product? What benefits have you realized?
Reducing the amount of physical computer hardware in the office (scanners, printers) through the use of software such as PDFfiller.
All-in-one tool that ultimately expedites the process of filling out and scanning forms.
What do you dislike?
Nothing in particular; fixed one-time pricing would be preferable over subscriptions though.
What problems are you solving with the product? What benefits have you realized?
Reducing the amount of physical computer hardware in the office (scanners, printers) through the use of software such as PDFfiller.


Get a powerful PDF editor for your Mac or Windows PC
Install the desktop app to quickly edit PDFs, create fillable forms, and securely store your documents in the cloud.

Edit and manage PDFs from anywhere using your iOS or Android device
Install our mobile app and edit PDFs using an award-winning toolkit wherever you go.

Get a PDF editor in your Google Chrome browser
Install the pdfFiller extension for Google Chrome to fill out and edit PDFs straight from search results.
List of extra features
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is a participant consent form?
A consent form is not simply about a person giving you permission to involve them in research, it is an agreement between the researcher and the research participant outlining the roles and responsibilities they are taking towards one another throughout the whole of the research process.
What is a research consent form?
Informed Consent is a voluntary agreement to participate in research. It is not merely a form that is signed but is a process, in which the subject has an understanding of the research and its risks. Informed consent is essential before enrolling a participant and ongoing once enrolled.
Do you need a consent form for surveys?
The informed consent process is a basic ethical obligation for researchers. The consent document is the only record linking the subject with the research. Most survey research meets the requirements for waivers of signed consent, because surveys conducted outside a research context rarely require written consent.
What does it mean if a researcher has obtained informed consent?
If a researcher has obtained informed consent from all his participants, it means that: they all understand the nature of the study and what will be asked of them. Researchers should try to avoid double-barreled questions, or questions that: ask about multiple issues.
What information should normally be disclosed to potential participants in research studies when their informed consent is obtain
For a valid consent, information provided to a research subject should include, but not limited to, health condition for which the research is proposed; nature and purpose/reason of the study; study treatment or intervention and experimental procedures; probable risks and benefits associated with research participation
Who informed consent?
The informed consent document is to be signed and dated by the participant or, when the participant is illiterate or physically unable, by a literate witness who ascertains that it was understood and confirms that consent was given freely.
What are the 4 principles of informed consent?
To discern the key components of informed consent, you need to understand the ethical issues of research involving human subjects. The principles of autonomy, beneficence, and justice are basic to these ethical issues and merit your consideration.
How do you get an informed consent?
The informed consent document should succinctly describe the research as it has been presented in the IRB application. Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I). Include a statement of agreement at the conclusion of the informed consent document.
What is ethical consent?
As an ethical doctrine, informed consent is a process of communication whereby a patient is enabled to make an informed and voluntary decision about accepting or declining medical care. Informed consent is an ethical concept that has become integral to contemporary medical ethics and medical practice.
Who is the person obtaining consent?
Consent is voluntary and freely given by the participant, guardian, or legally-authorized representative. Consent form is signed and dated by a person other than the participant or the participant's guardian or legally-authorized representative who can attest that the requirements for informed consent has been met.
Does a physician have to sign an informed consent?
True informed consent is a process of managing a patient's expectations; it is not just a signature on a document. The physician must then provide sufficient information to the patient so that a reasonable and informed decision regarding a treatment plan can be made. This physician responsibility cannot be delegated.
How will you obtain consent from participants?
Obtaining consent involves informing the subject about his or her rights, the purpose of the study, the procedures to be undergone, and the potential risks and benefits of participation. Subjects in the study must participate willingly. Vulnerable populations (i.e. prisoners, children, pregnant women, etc.)
What is obtained consent?
Obtaining consent involves explaining the research and assessing participant comprehension using a consent document, usually a written consent form or information sheet, as a guide for the verbal explanation of the study.
What is a consent form?
a form signed by a patient prior to a medical procedure to confirm that he or she agrees to the procedure and is aware of any risks that might be involved. The primary purpose of the consent form is to provide evidence that the patient gave consent to the procedure in question.
Can you give medical consent over the phone?
Oral Consent Scripts The oral consent script may be used when obtaining informed consent in person, or may be used over the telephone.
eSignature workflows made easy
Sign, send for signature, and track documents in real-time with signNow.