Consent Signature Request For Free
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent

Upload your document to the PDF editor

Type anywhere or sign your form

Print, email, fax, or export

Try it right now! Edit pdf
Users trust to manage documents on pdfFiller platform
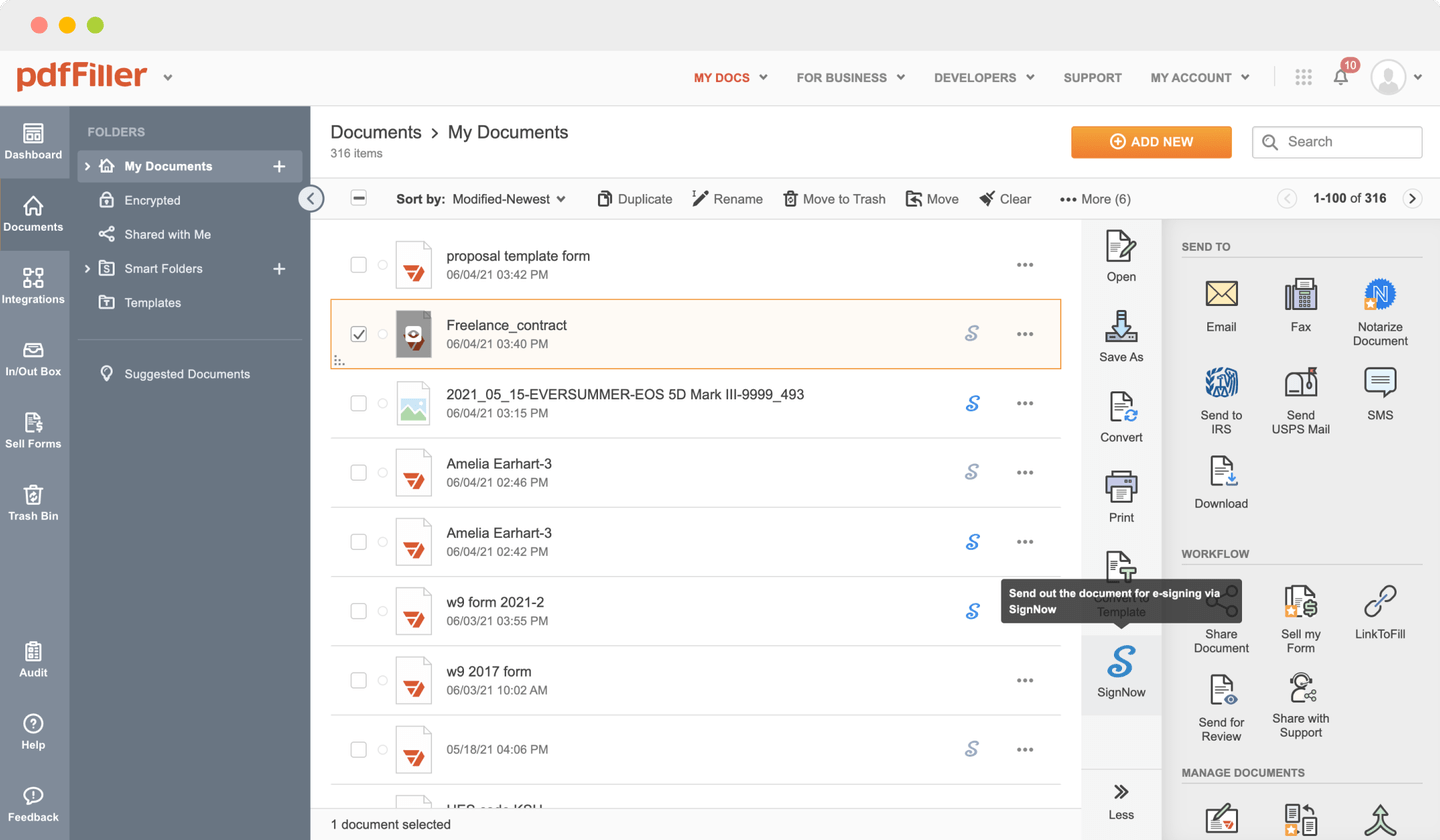
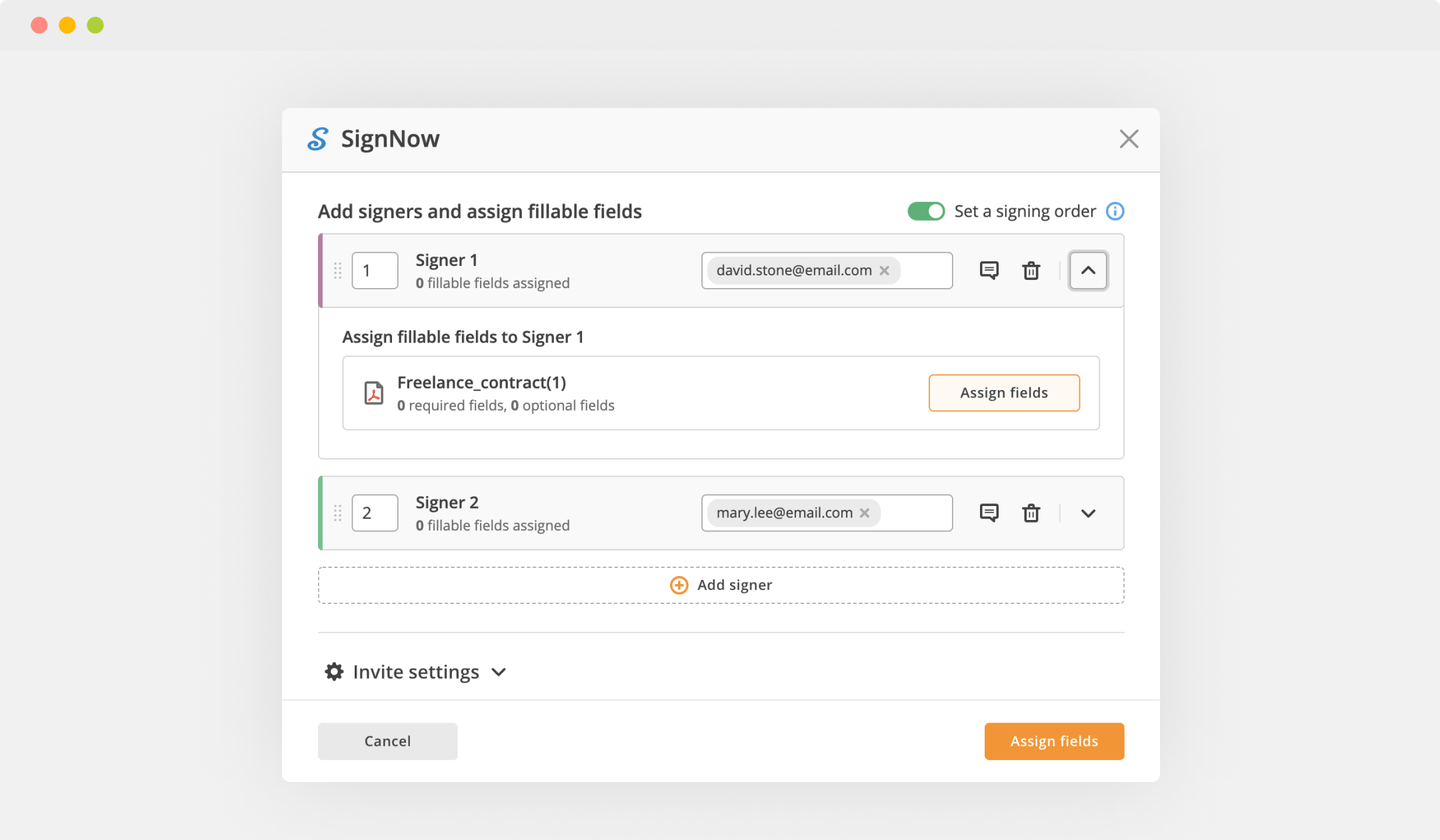
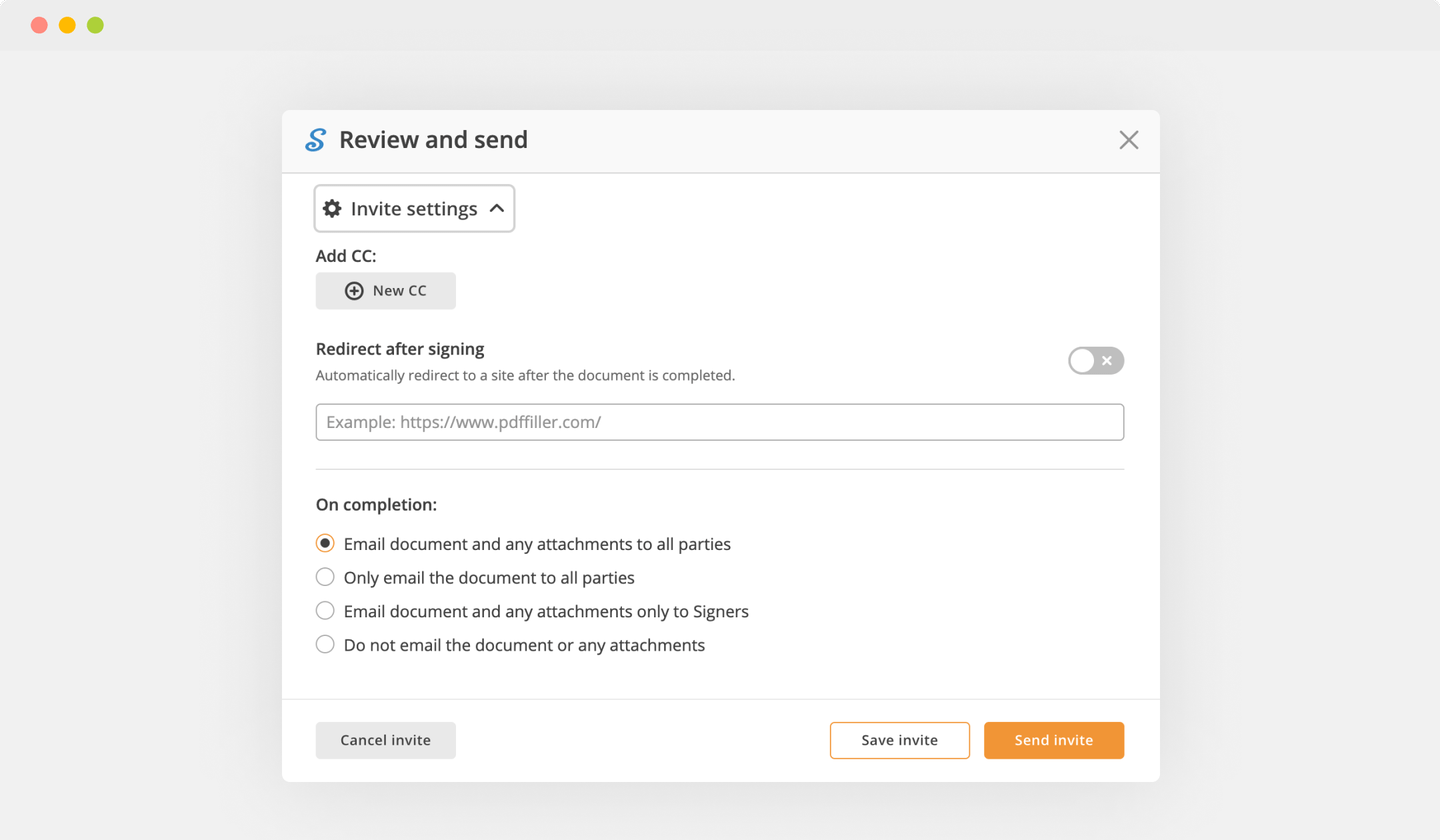
Send documents for eSignature with signNow
Create role-based eSignature workflows without leaving your pdfFiller account — no need to install additional software. Edit your PDF and collect legally-binding signatures anytime and anywhere with signNow’s fully-integrated eSignature solution.
All-in-one PDF software
A single pill for all your PDF headaches. Edit, fill out, eSign, and share – on any device.
pdfFiller scores top ratings in multiple categories on G2
How to Consent Signature Request
Stuck with numerous applications for managing documents? Try our solution instead. Use our tool to make the process fast and simple. Create document templates completely from scratch, modify existing forms, integrate cloud services and utilize even more features without leaving your browser. You can Consent Signature Request directly, all features, like orders signing, reminders, attachment and payment requests, are available instantly. Have a major advantage over those using any other free or paid applications.
How-to Guide
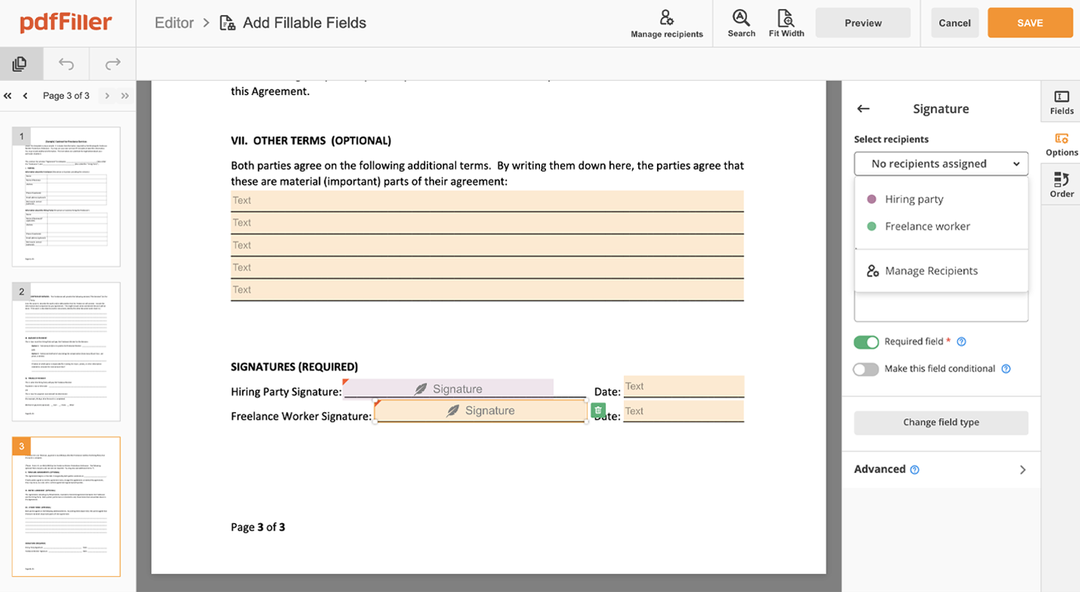
How to edit a PDF document using the pdfFiller editor:
01
Upload your form to pdfFiller
02
Find the Consent Signature Request feature in the editor's menu
03
Make all the necessary edits to the file
04
Push the orange “Done" button at the top right corner
05
Rename the template if it's necessary
06
Print, email or save the file to your desktop
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
LEIGH K
2014-09-07
JUST HOPE THE INSURANCE COMPANIES WILL ACCEPT THIS FORM AND PAY US. WISH IT HAD A CAPABILITY OF ALIGNING ALL LINE HORIZONTALLY SO IT DOESN'T LOOK SO HAPHAZARD

Gene W
2019-04-11
It's probably just me but it's hard to figure out what does what.Also trying to figure out what I can move on a PDF and what I have to erase and fill-in again. Otherwise I think it's one of the best. I should also say seems be working with my Dragon NaturallySpeaking


Get a powerful PDF editor for your Mac or Windows PC
Install the desktop app to quickly edit PDFs, create fillable forms, and securely store your documents in the cloud.

Edit and manage PDFs from anywhere using your iOS or Android device
Install our mobile app and edit PDFs using an award-winning toolkit wherever you go.

Get a PDF editor in your Google Chrome browser
Install the pdfFiller extension for Google Chrome to fill out and edit PDFs straight from search results.
List of extra features
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Who is the person obtaining consent?
Consent is voluntary and freely given by the participant, guardian, or legally-authorized representative. Consent form is signed and dated by a person other than the participant or the participant's guardian or legally-authorized representative who can attest that the requirements for informed consent has been met.
What is obtained consent?
Obtaining consent involves explaining the research and assessing participant comprehension using a consent document, usually a written consent form or information sheet, as a guide for the verbal explanation of the study.
What is a participant consent form?
A consent form is not simply about a person giving you permission to involve them in research, it is an agreement between the researcher and the research participant outlining the roles and responsibilities they are taking towards one another throughout the whole of the research process.
What is a research consent form?
Informed Consent is a voluntary agreement to participate in research. It is not merely a form that is signed but is a process, in which the subject has an understanding of the research and its risks. Informed consent is essential before enrolling a participant and ongoing once enrolled.
Do you need a consent form for surveys?
The informed consent process is a basic ethical obligation for researchers. The consent document is the only record linking the subject with the research. Most survey research meets the requirements for waivers of signed consent, because surveys conducted outside a research context rarely require written consent.
How is informed consent obtained?
Informed Consent is a voluntary agreement to participate in research. Obtaining consent involves informing the subject about his or her rights, the purpose of the study, the procedures to be undergone, and the potential risks and benefits of participation. Subjects in the study must participate willingly.
What are the 4 principles of informed consent?
To discern the key components of informed consent, you need to understand the ethical issues of research involving human subjects. The principles of autonomy, beneficence, and justice are basic to these ethical issues and merit your consideration.
When gaining informed consent from participants in a research study you should definitely?
Question: When Gaining Informed Consent From Participants In A Research Study, You Should Definitely Make Sure You Inform The Participant That They Can Quit Participating In The Study At Any Time Without Any Fear Of Retaliation (to Avoid Coercion).
What information should normally be disclosed to potential participants in research studies when their informed consent is obtain
For a valid consent, information provided to a research subject should include, but not limited to, health condition for which the research is proposed; nature and purpose/reason of the study; study treatment or intervention and experimental procedures; probable risks and benefits associated with research participation
Who can sign an informed consent form?
Generally, you are responsible for: Ensuring that the consent form is signed by the appropriate person.g., the patient, the guardian, the agent under a durable attorney for health care. Your only role is as a witness to the person putting his or her signature on the form and dating the form.
Does inform consent require a signature?
At minimum, it involves obtaining the signature of the participant (or the legally-authorized representative or parent(s), when approved) as well as the person obtaining consent. In most cases, the federal regulations require that informed consent be documented, but they also provide some important exceptions.
Why a signed informed consent form is necessary?
Informed consent is essential before enrolling a participant and ongoing once enrolled. The goal of the informed consent process is to provide sufficient information so that a participant can make an informed decision about whether to enroll in a study or to continue participation.
Can nurses give informed consent?
Informed consent should be a collaborative activity between the physician, nurse, and patient. The physician should have obtained consent before the nurse has the patient sign a form. Nurses can offer what we do best patient teaching, as we check patient understanding and obtain written consent.
Can a PA obtain informed consent?
The Supreme Court ruled that a physician's duty to provide information to a patient sufficient to obtain her informed consent is non-renewable a physician assistant may not provide any aspect of informed consent to a patient.
What constitutes informed consent?
Medical Definition of Informed consent: The process by which a patient learns about and understands the purpose, benefits, and potential risks of a medical or surgical intervention, including clinical trials, and then agrees to receive the treatment or participate in the trial.
eSignature workflows made easy
Sign, send for signature, and track documents in real-time with signNow.