ESign Clinical Trial Agreement Template For Free




Users trust to manage documents on pdfFiller platform
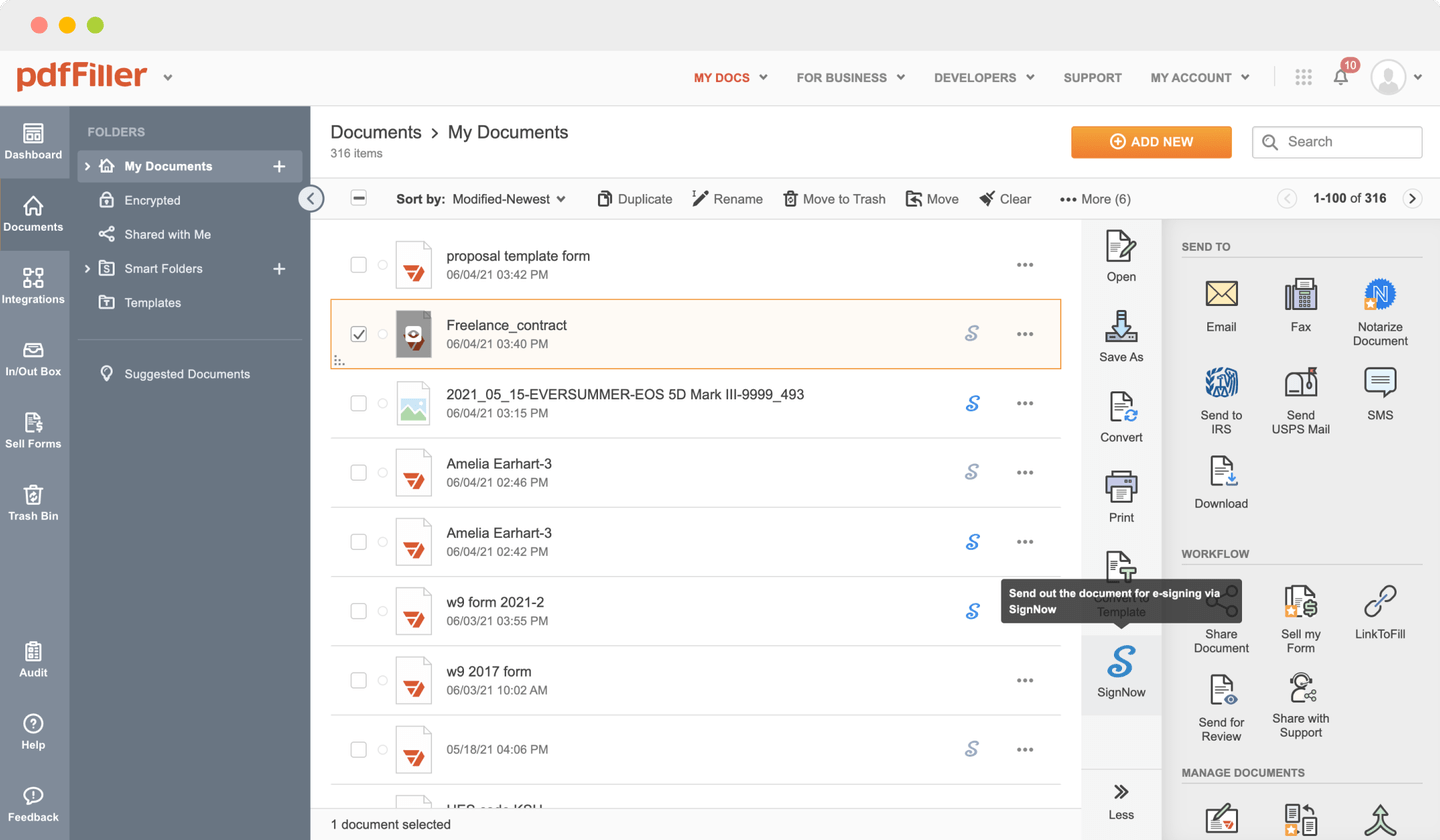
Send documents for eSignature with signNow
Watch a quick video tutorial on how to ESign Clinical Trial Agreement Template
pdfFiller scores top ratings in multiple categories on G2
ESign Clinical Trial Agreement Template with the swift ease
pdfFiller allows you to ESign Clinical Trial Agreement Template in no time. The editor's handy drag and drop interface ensures quick and intuitive signing on any operaring system.
Signing PDFs online is a quick and secure way to verify documents anytime and anywhere, even while on the fly.
See the detailed guide on how to ESign Clinical Trial Agreement Template online with pdfFiller:
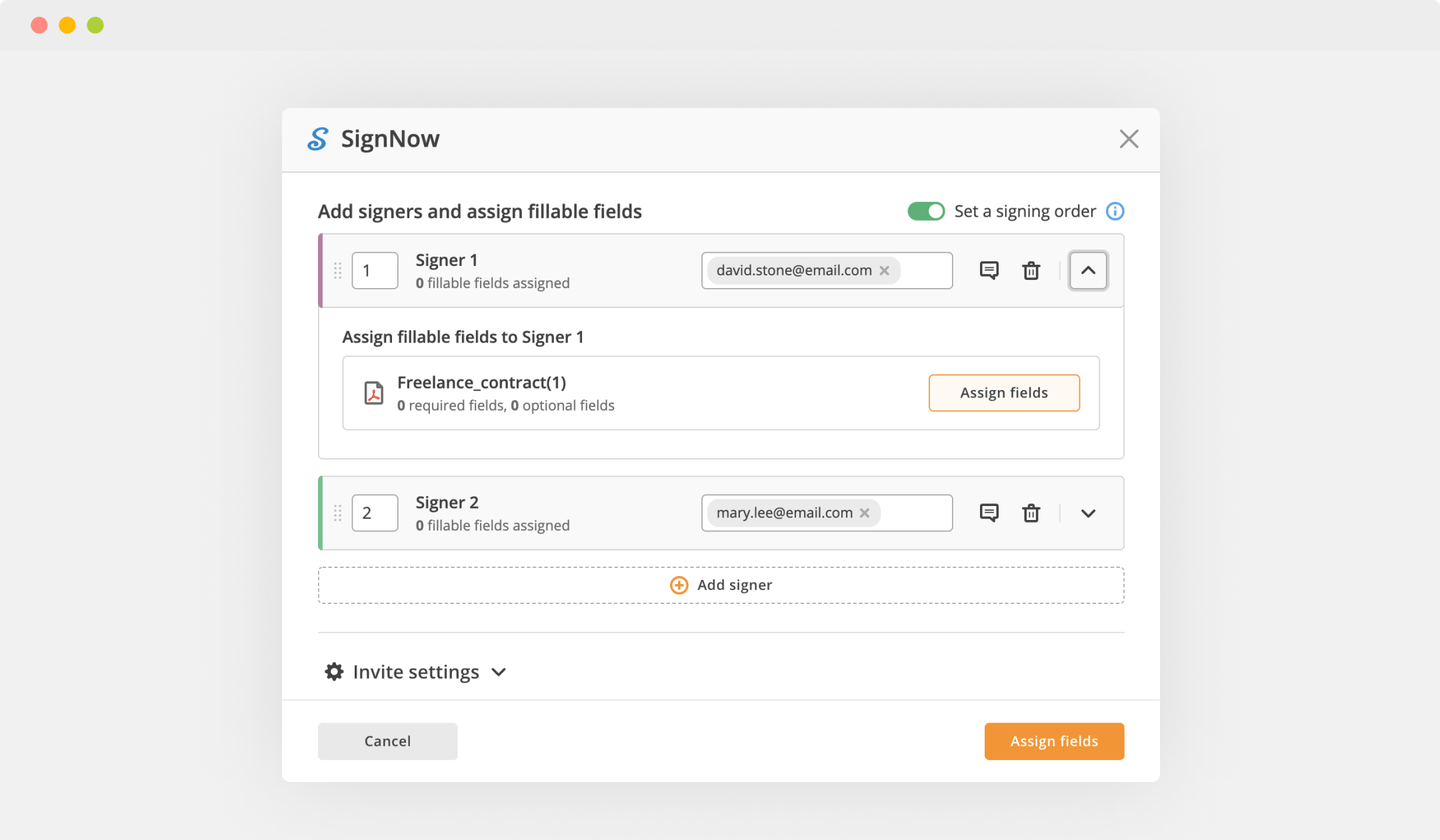
Add the form you need to sign to pdfFiller from your device or cloud storage.
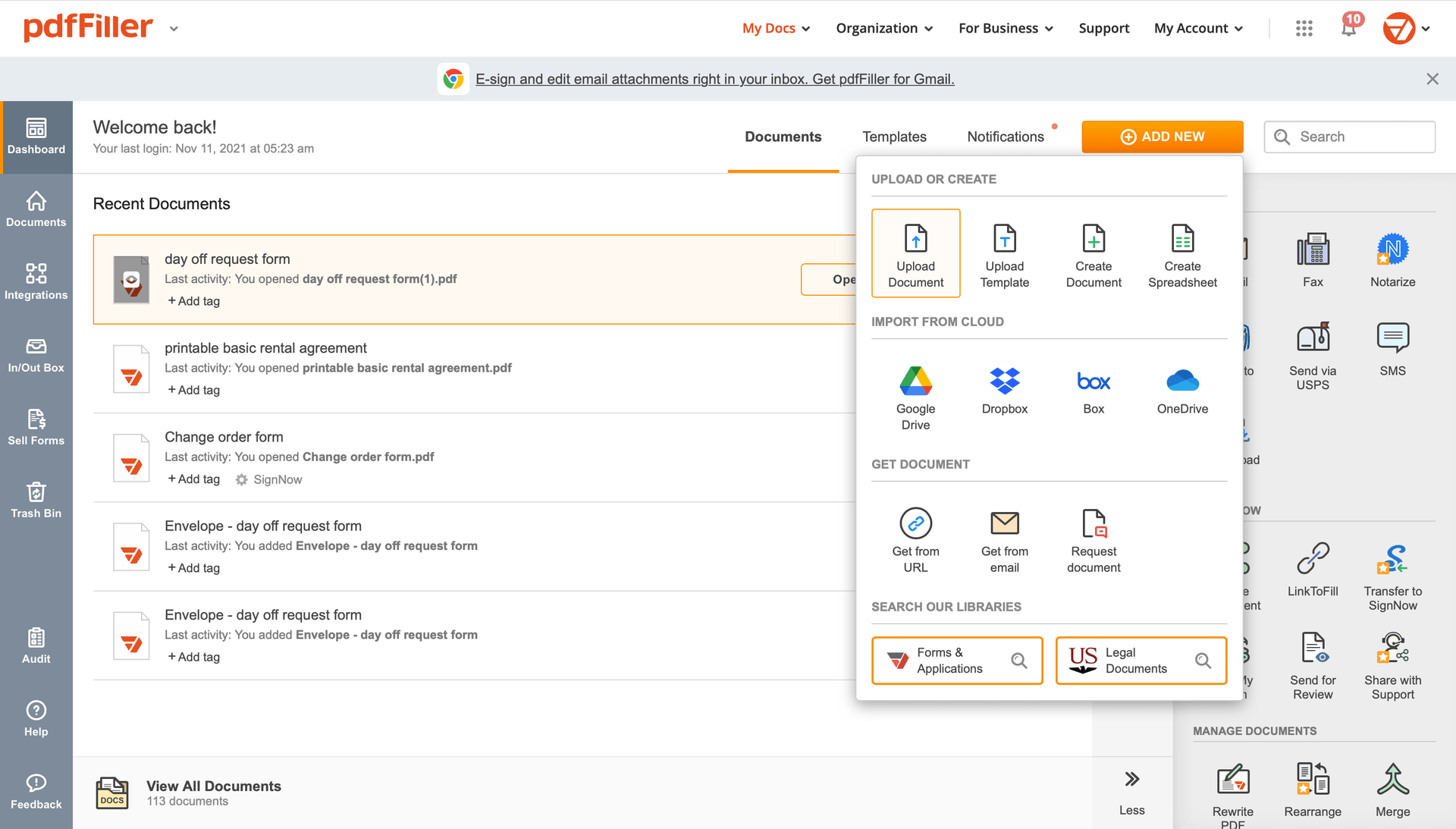
Once the document opens in the editor, hit Sign in the top toolbar.
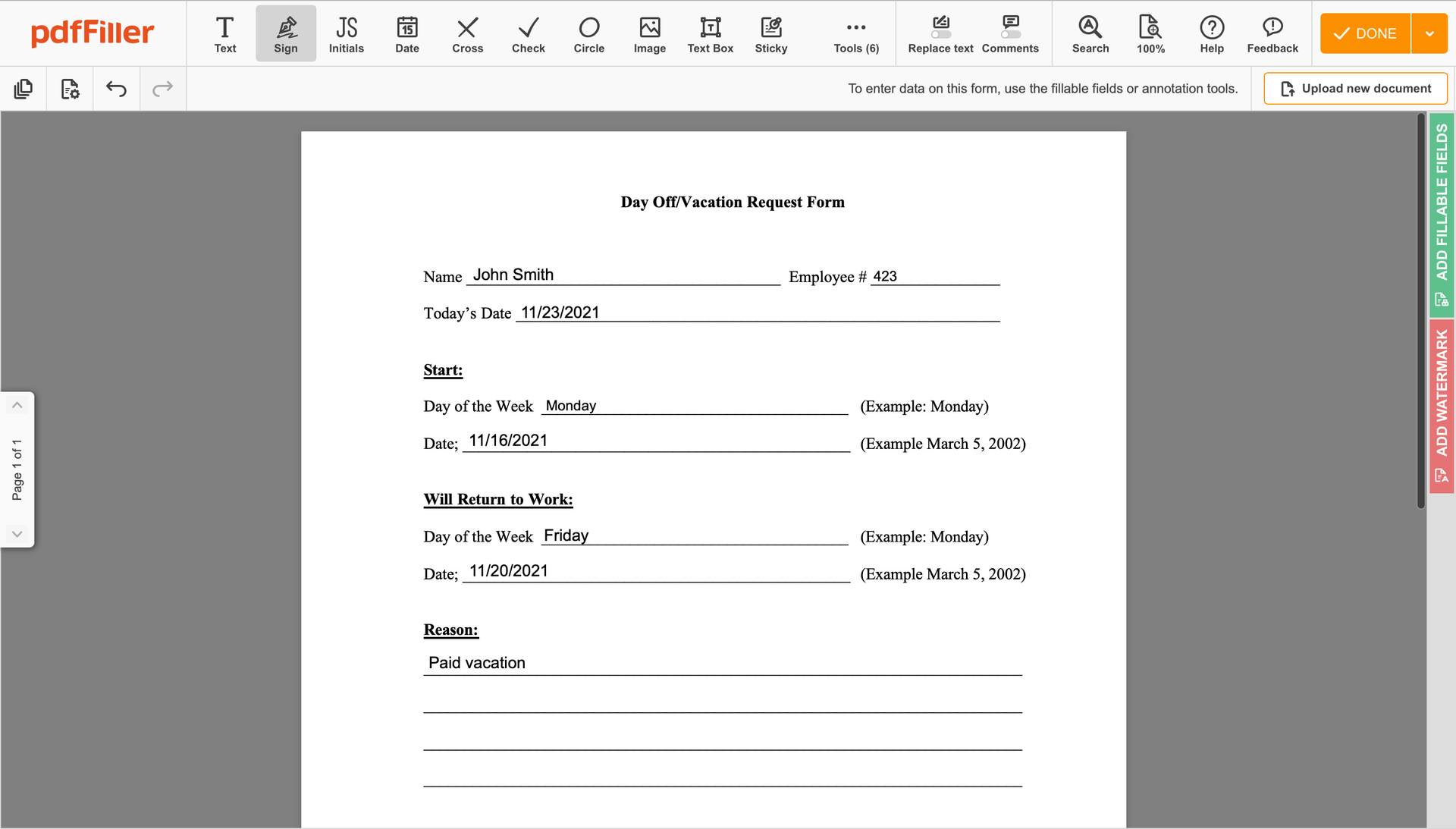
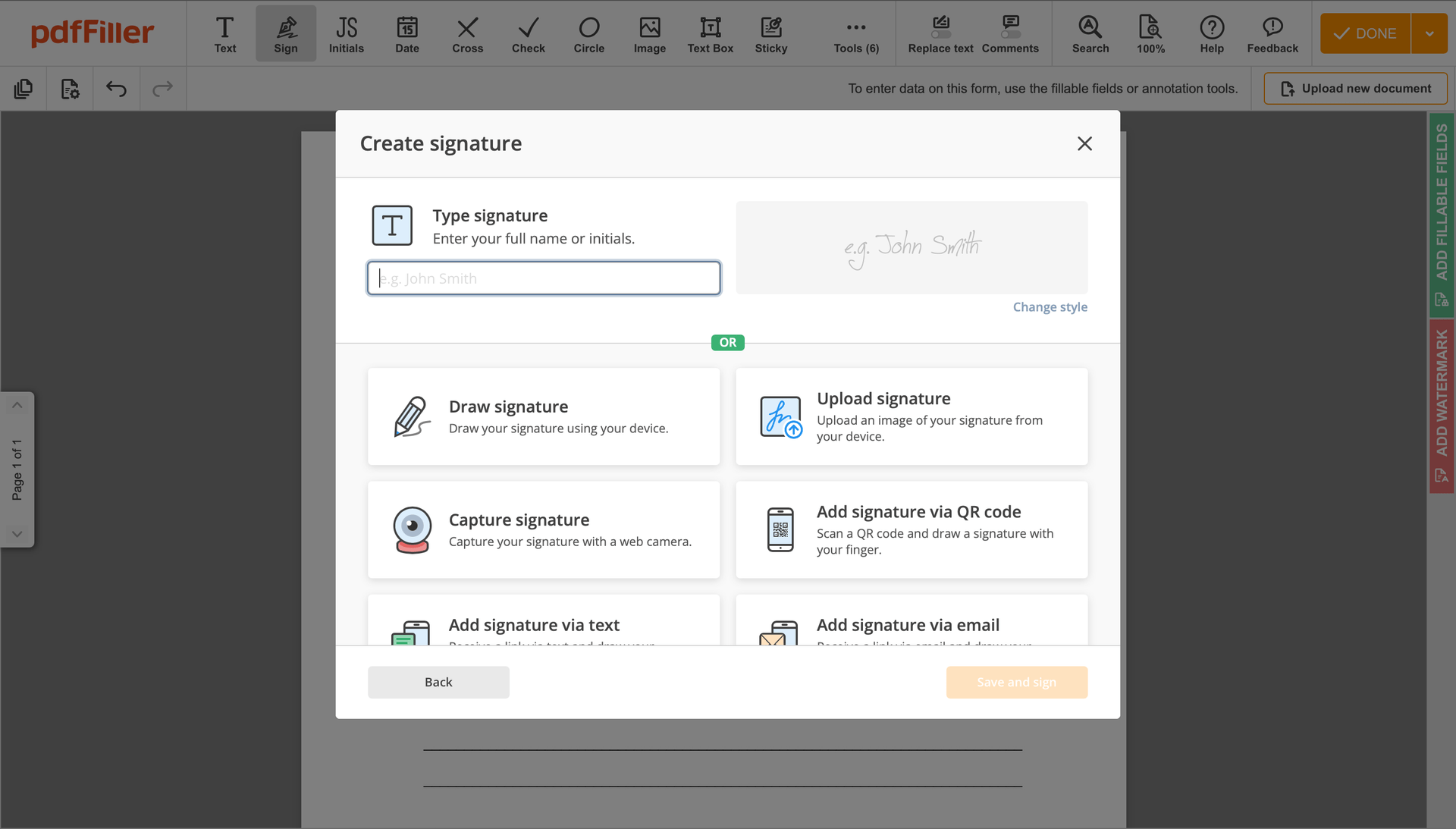
Create your electronic signature by typing, drawing, or uploading your handwritten signature's photo from your laptop. Then, click Save and sign.
Click anywhere on a document to ESign Clinical Trial Agreement Template. You can move it around or resize it using the controls in the hovering panel. To use your signature, hit OK.
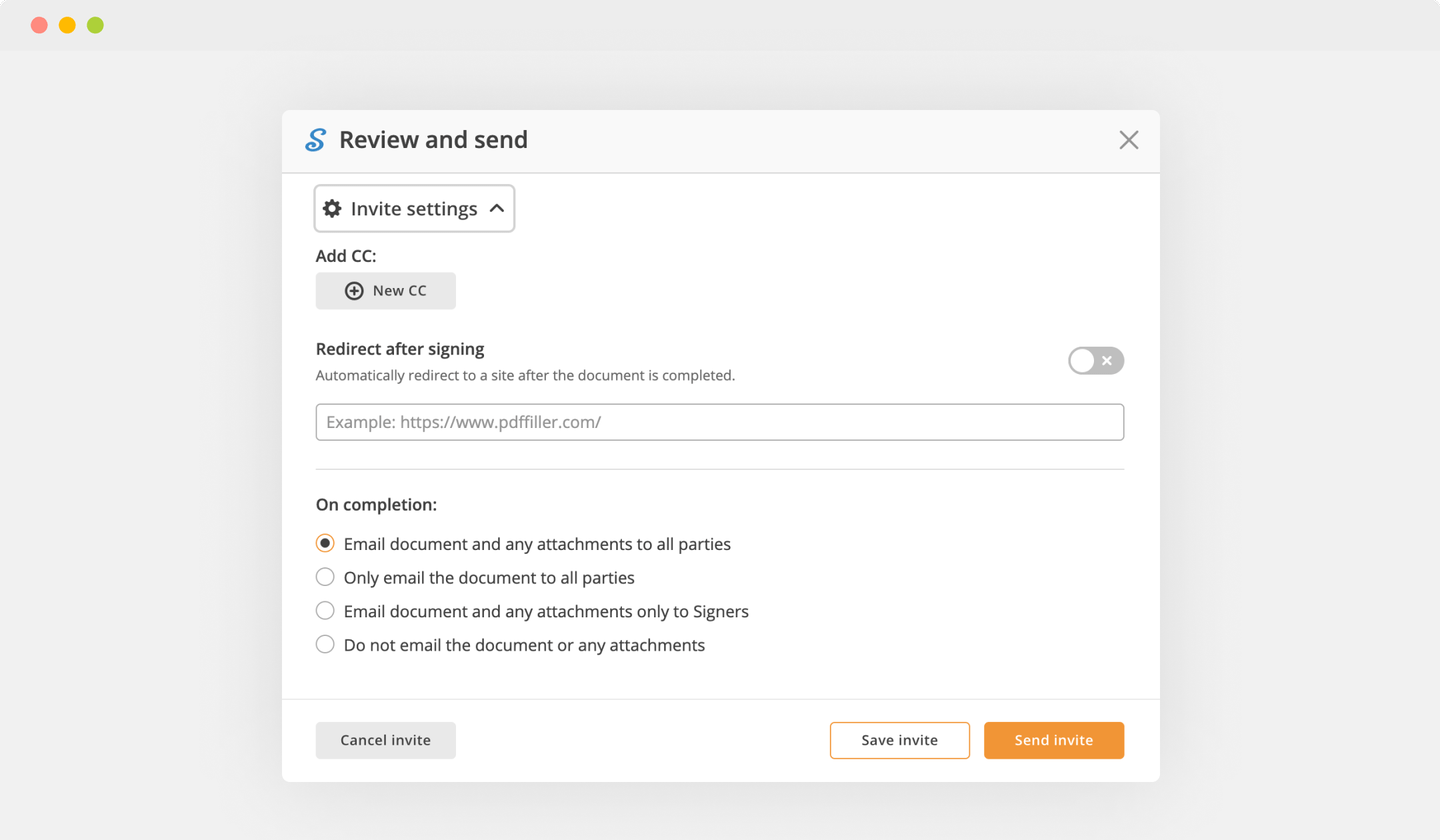
Complete the signing session by hitting DONE below your document or in the top right corner.
Next, you'll return to the pdfFiller dashboard. From there, you can download a completed copy, print the document, or send it to other people for review or validation.
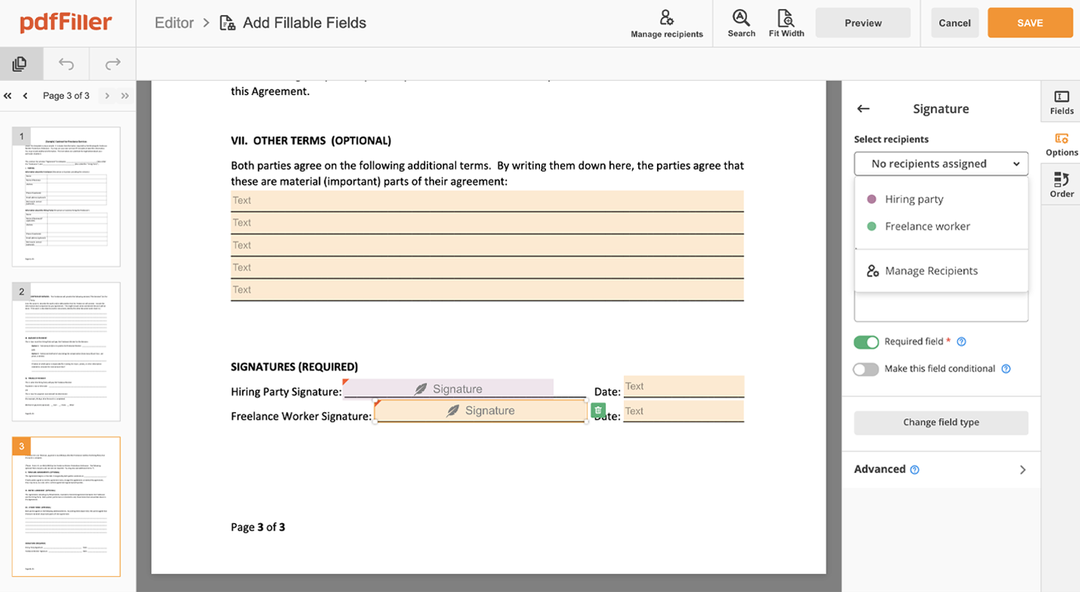
Still using numerous programs to create and sign your documents? Use our all-in-one solution instead. Use our editor to make the process efficient. Create document templates completely from scratch, edit existing forms, integrate cloud services and utilize even more features without leaving your account. Plus, it enables you to use design Clinical Trial Agreement Template and add other features like signing orders, alerts, attachment and payment requests, easier than ever. Get the value of full featured tool, for the cost of a lightweight basic app.
How to edit a PDF document using the pdfFiller editor:
How to Send a PDF for eSignature
What our customers say about pdfFiller