Lock Signature Accreditation मुफ़्त में
Create a legally-binding electronic signature and add it to contracts, agreements, PDF forms, and other documents – regardless of your location. Collect and track signatures with ease using any device.
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.

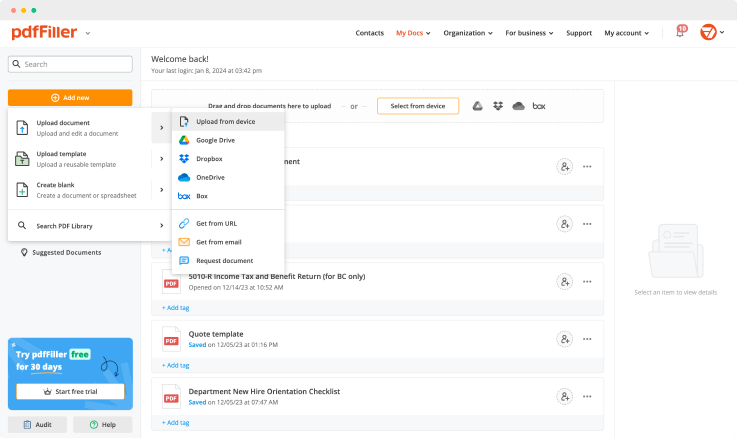
Upload a document

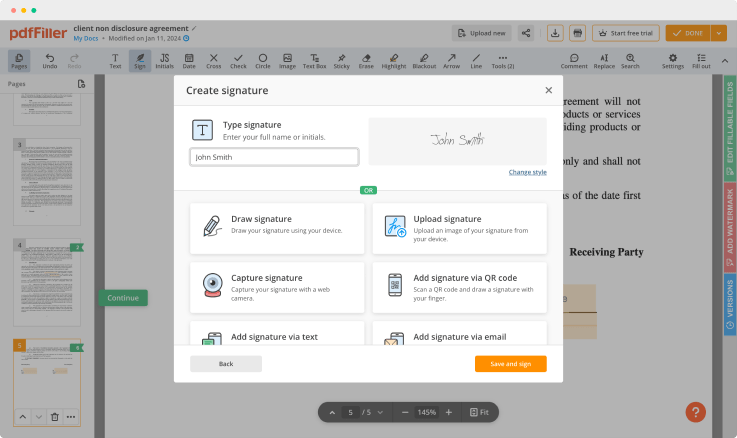
Generate your customized signature

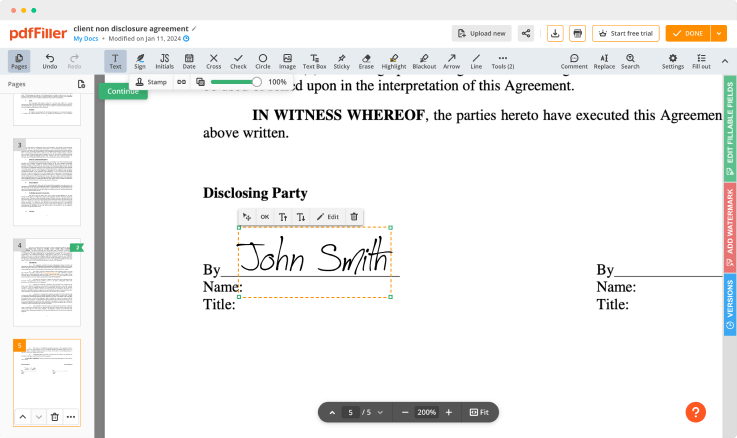
Adjust the size and placement of your signature

Download, share, print, or fax your signed document
Join the world’s largest companies
Employees at these companies use our products.

pdfFiller scores top ratings in multiple categories on G2
4.6/5
— from 710 reviews








Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution
Upload your document to pdfFiller and open it in the editor.

Unlimited document storage
Generate and save your electronic signature using the method you find most convenient.

Widely recognized ease of use
Resize your signature and adjust its placement on a document.

Reusable templates & forms library
Save a signed, printable document on your device in the format you need or share it via email, a link, or SMS. You can also instantly export the document to the cloud.
The benefits of electronic signatures
Bid farewell to pens, printers, and paper forms.

Efficiency
Enjoy quick document signing and sending and reclaim hours spent on paperwork.

Accessibility
Sign documents from anywhere in the world. Speed up business transactions and close deals even while on the go.

Cost savings
Eliminate the need for paper, printing, scanning, and postage to significantly cut your operational costs.

Security
Protect your transactions with advanced encryption and audit trails. Electronic signatures ensure a higher level of security than traditional signatures.

Legality
Electronic signatures are legally recognized in most countries around the world, providing the same legal standing as a handwritten signature.

Sustainability
By eliminating the need for paper, electronic signatures contribute to environmental sustainability.
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance
Regulates the use and holding of personal data belonging to EU residents.

SOC 2 Type II Certified
Guarantees the security of your data & the privacy of your clients.

PCI DSS certification
Safeguards credit/debit card data for every monetary transaction a customer makes.

HIPAA compliance
Protects the private health information of your patients.

CCPA compliance
Enhances the protection of personal data and the privacy of California residents.
Lock Signature Accreditation Feature
Unlock a new level of security and reliability with the Lock Signature Accreditation feature. This tool provides assurance that your documents and communications are safe and authentic.
Key Features
Ensures document authenticity and integrity
Provides a digital signature for legal verification
Offers audit trails for all signed documents
Integrates seamlessly with existing systems
Supports multiple file formats for versatility
Potential Use Cases and Benefits
Legal documents that require formal signatures
Contracts in business transactions
Secure communications in financial services
Verification of identity in healthcare records
Protection of intellectual property with secure signatures
The Lock Signature Accreditation feature solves your security concerns by offering a reliable way to validate documents. With this feature, you can easily sign and verify documents, ensuring that your transactions are trustworthy and legally binding. Whether you handle sensitive information or engage in important contracts, this feature gives you the confidence you need to operate securely.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
What does it mean to be 21 CFR Part 11 compliant?
FDA 21 CFR Part 11 compliance dictates that electronic records that are signed must contain a name, the signature meaning and the date/time of signing. ... 21 CFR compliance dictates that an FDA-regulated company's electronic system must be validated according to the FDA's validation standards.
What are 21 CFR Part 11 requirements?
Part 11, as it is commonly called, defines the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records (Title 21 CFR Part 11 Section 11.1 (a)).
Does 21 CFR part 11 apply to medical devices?
Medical device companies that wish to sell their devices in the US and EU must implement a quality management system that meets the requirements of 21 CFR Part 820 and ISO 13485:2016. ... Specifically, 21 CFR Part 11, the FDA's regulations for electronic documentation and electronic signatures.
What is 21 CFR in pharma PDF?
The CFR contains 50 titles. CFR: Short for Code of Federal Regulations, which is a coded (numbers and letters) set of laws published by the federal government of the United States. Part 11: Scope is specific to electronic records and electronic signatures, which includes electronic submissions to the FDA.
What are the predicate rules for Part 11?
The predicate rules mandate what records must be maintained; the content of records; whether signatures are required; how long records must be maintained, etc. If there is no FDA requirement that a particular record be created or retained, then 21 CFR Part 11 most likely does not apply to the record.
Ready to try pdfFiller's? Lock Signature Accreditation मुफ़्त में
Upload a document and create your digital autograph now.