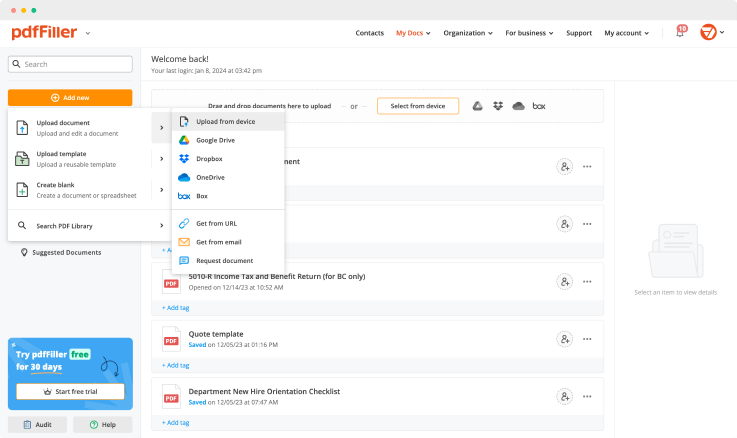
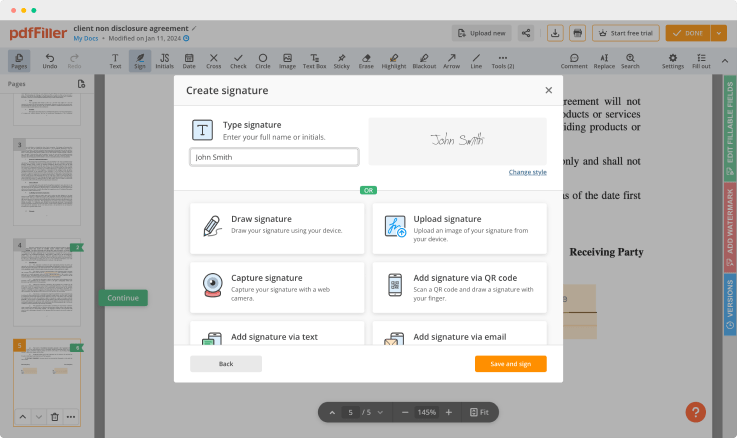
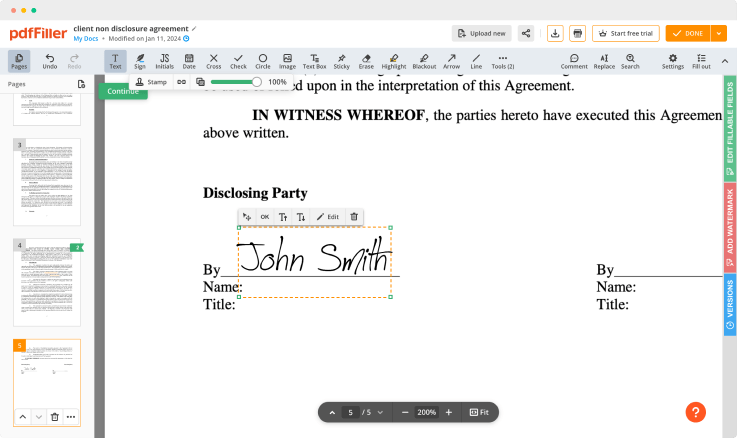
Regulate Formula Record For Free




Join the world’s largest companies









Why choose pdfFiller for eSignature and PDF editing?

Cross-platform solution

Unlimited document storage

Widely recognized ease of use

Reusable templates & forms library
The benefits of electronic signatures

Efficiency

Accessibility

Cost savings

Security

Legality

Sustainability
Enjoy straightforward eSignature workflows without compromising data security

GDPR compliance

SOC 2 Type II Certified

PCI DSS certification

HIPAA compliance

CCPA compliance
Regulate Formula Record Feature
The Regulate Formula Record feature offers a comprehensive solution for managing and tracking your formulas with ease. With this tool, you gain control over your records, ensuring accuracy and efficiency in your process.
Key Features
Potential Use Cases and Benefits
This feature helps solve your challenges by centralizing your formula records. You no longer have to worry about lost or outdated information. With Regulate Formula Record, you maintain accuracy, save time, and simplify your workflow. Discover peace of mind as you focus on what truly matters.
Instructions and Help about Regulate Formula Record For Free
Regulate Formula Record: easy document editing
The Portable Document Format or PDF is a widely used document format for a variety of reasons. PDFs are accessible from any device, so you can share them between devices with different screen resolution and settings. It will appear the same no matter you open it on Mac computer or an Android smartphone.
The next point is security: PDF files are easy to encrypt, so it's safe to share any personal data in them from person to person. That’s why it’s important to choose a secure editing tool for managing documents. Some platforms give you access to an opening history to track down people who read or filled out the document before without your notice.
pdfFiller is an online editor that allows to create, modify, sign, and share PDF using one browser tab. Thanks to the numerous integrations with the popular solutions for businesses, you can upload an information from any system and continue where you left off. Once you finish changing a document, mail it to recipients to fill out, and you'll get a notification when they're finished.
Use powerful editing tools to type in text, annotate and highlight. Once a document is completed, download it to your device or save it to the third-party integration cloud. Add images to your PDF and edit its appearance. Collaborate with others to fill out the document and request an attachment if needed. Add fillable fields and send documents for signing. Change a template’s page order.
Get your documents completed in four simple steps:
For pdfFiller’s FAQs
Ready to try pdfFiller's? Regulate Formula Record