
Get the free dea controlled substance inventory form - wiki umms med umich
Show details
University of Michigan Controlled Substance Research Records DEA Biennial Controlled Substance Inventory Form Date DEA Registrant Print Name DEA Registration State of MI Controlled Substance permanent ID site specific Inventory Performed by Print Name Signature Inventory Witness Start of day End of day DEA Schedule Page Controlled Substance Container Unit Type Quantity Volume ml Concentration mg/ml Schedule I and II drugs must be separated from all other drugs or placed on a separate form. of...
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign dea controlled substance inventory
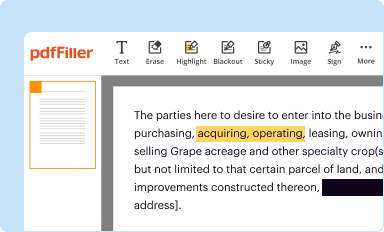
Edit your dea controlled substance inventory form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
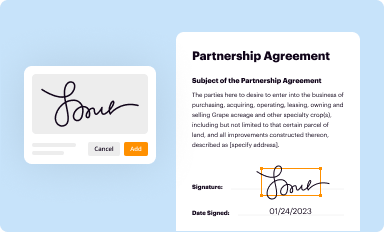
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your dea controlled substance inventory form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing dea controlled substance inventory online
To use our professional PDF editor, follow these steps:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit dea controlled substance inventory. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
Dealing with documents is simple using pdfFiller. Now is the time to try it!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out dea controlled substance inventory
How to fill out controlled substance inventory sheet:
01
Start by gathering all the necessary information about the controlled substances you have in your inventory, including their names, strengths, quantities, and expiration dates.
02
Next, open the controlled substance inventory sheet and locate the designated sections for recording the required information.
03
Begin filling out the inventory sheet by entering the name of each controlled substance in the appropriate column. Make sure to write the names accurately to avoid any confusion.
04
In the corresponding column, specify the strength of each controlled substance. This information is often indicated on the medication label or packaging.
05
Record the quantity of each controlled substance you currently have in your inventory. This can be done by counting the number of units or measuring the quantity as specified (e.g., milligrams, milliliters, etc.).
06
Check the expiration dates for each controlled substance and record them accordingly. It is crucial to monitor and manage the expiration dates to ensure the safe and effective use of the substances.
07
Review your entries on the controlled substance inventory sheet for accuracy and completeness. Make any necessary corrections or additions before finalizing the document.
08
Finally, store the completed controlled substance inventory sheet in a secure and accessible location for future reference and potential inspections.
Who needs controlled substance inventory sheet:
01
Healthcare facilities such as hospitals, clinics, and pharmacies that stock and dispense controlled substances are required to maintain a controlled substance inventory sheet.
02
Practitioners who are authorized to prescribe and administer controlled substances, such as physicians, dentists, veterinarians, and other healthcare professionals, need to keep a record of the controlled substances they possess.
03
Regulatory bodies, law enforcement agencies, and auditors may request to review the controlled substance inventory sheet to ensure compliance with legal and regulatory requirements.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send dea controlled substance inventory to be eSigned by others?
When you're ready to share your dea controlled substance inventory, you can swiftly email it to others and receive the eSigned document back. You may send your PDF through email, fax, text message, or USPS mail, or you can notarize it online. All of this may be done without ever leaving your account.
How can I edit dea controlled substance inventory on a smartphone?
You can do so easily with pdfFiller’s applications for iOS and Android devices, which can be found at the Apple Store and Google Play Store, respectively. Alternatively, you can get the app on our web page: https://edit-pdf-ios-android.pdffiller.com/. Install the application, log in, and start editing dea controlled substance inventory right away.
Can I edit dea controlled substance inventory on an iOS device?
Use the pdfFiller mobile app to create, edit, and share dea controlled substance inventory from your iOS device. Install it from the Apple Store in seconds. You can benefit from a free trial and choose a subscription that suits your needs.
What is DEA controlled substance inventory?
The DEA controlled substance inventory is a comprehensive record maintained by practitioners and institutions to track the quantity of controlled substances on hand. It helps ensure compliance with federal regulations regarding the storage and use of these drugs.
Who is required to file DEA controlled substance inventory?
Practitioners, pharmacies, hospitals, and other entities that handle controlled substances are required to file a DEA controlled substance inventory to comply with federal regulations.
How to fill out DEA controlled substance inventory?
To fill out a DEA controlled substance inventory, you need to provide the date of the inventory, the name of the controlled substance, its strength, the dosage form, the quantity on hand, and the signature of the person conducting the inventory.
What is the purpose of DEA controlled substance inventory?
The purpose of the DEA controlled substance inventory is to maintain accurate records for accountability, detect potential diversion or misuse, and ensure compliance with federal and state regulations concerning controlled substances.
What information must be reported on DEA controlled substance inventory?
The information that must be reported includes the date of the inventory, the names and concentrations of each controlled substance, the quantities on hand, and the signature of the individual conducting the inventory.
Fill out your dea controlled substance inventory online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Dea Controlled Substance Inventory is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.






















