Get the free GMP #147 - Virginia Department of Health - Commonwealth of Virginia - vdh virginia
Show details
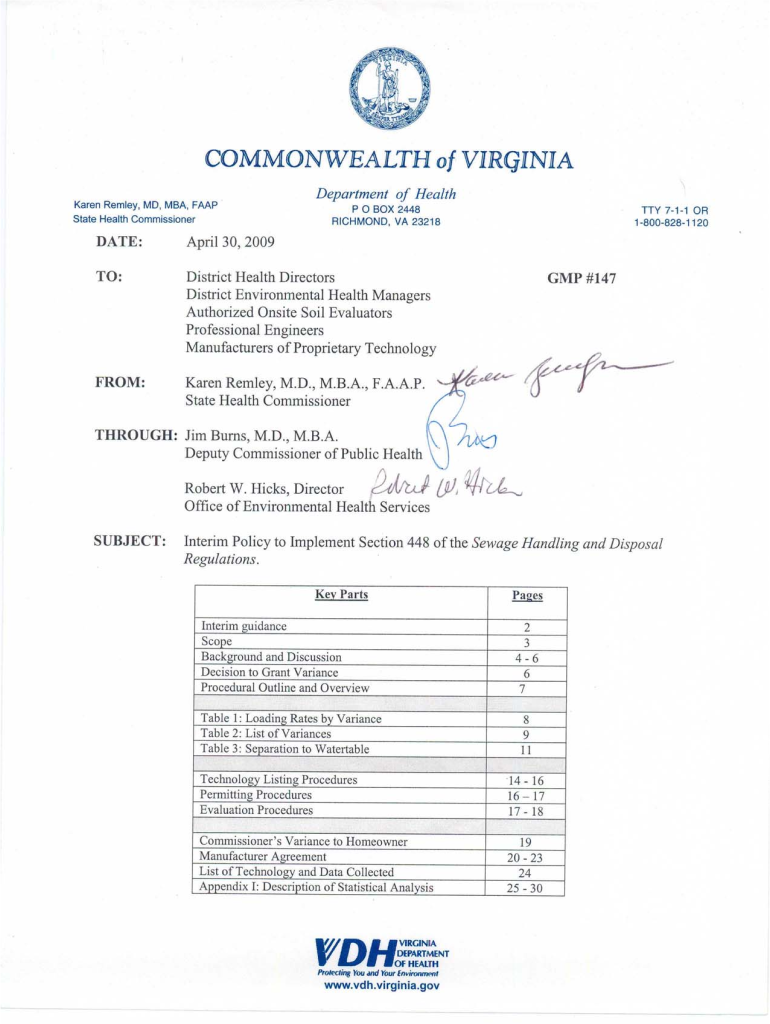
30 Apr 2009 ... District Environmental Health Managers ... Interim Policy to Implement Section 448 of the Sewage Handling ... www.vdh.virginia.gov ... Pursuant to HITS and 881276 of the 2009 General
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign gmp 147 - virginia

Edit your gmp 147 - virginia form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your gmp 147 - virginia form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit gmp 147 - virginia online
To use our professional PDF editor, follow these steps:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit gmp 147 - virginia. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
Fill
form
: Try Risk Free






People Also Ask about
What are the 5 principles of GMP?
Good manufacturing practices (GMPs) help to ensure the consistent quality and safety of products by focusing attention on five key elements, which are often referred to as the 5 P's of GMP—people, premises, processes, products and procedures (or paperwork).
What does GMP stand for in food safety?
program. These include: Good Manufacturing Practices (GMPs) Good Manufacturing Practices* (GMPs) are the basic operational and environmental conditions required to produce safe foods.
What is GMP Pharma standard?
GMP defines quality measures for both production and quality control and defines general measures to ensure that processes necessary for production and testing are clearly defined, validated, reviewed, and documented, and that the personnel, premises and materials are suitable for the production of pharmaceuticals and
What does GMP mean in quality assurance?
Good Manufacturing Practice (GMP) deals with quality measures for both production and quality control as part of quality assurance. GMP also ensures that processes should be clearly defined, approved, revised, and recorded for production and testing.
What are the 5 key components of GMP?
5 Main Components of GMP Products. People. Processes. Procedures. Premises.
What are the 10 basic principles of GMP?
Principles of GMP 10 Principle of Good Manufacturing Practises. PRINCIPLE 1: Step by step written procedures. PRINCIPLE 2: Follow procedures. PRINCIPLE 3 Document work. PRINCIPLE 4 Validate work. PRINCIPLE 5 Integrate productivity, quality & safety into facilities & equipment. PRINCIPLE 6 Maintain facilities & equipment.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is gmp 147 - virginia?
GMP 147 - Virginia is a form used to report gross receipts for Virginia state tax purposes.
Who is required to file gmp 147 - virginia?
Businesses operating in Virginia that have gross receipts exceeding a certain threshold are required to file GMP 147 - Virginia.
How to fill out gmp 147 - virginia?
GMP 147 - Virginia can be filled out online through the Virginia Department of Taxation's website or by mailing in a paper form.
What is the purpose of gmp 147 - virginia?
The purpose of GMP 147 - Virginia is to report gross receipts for tax assessment and compliance.
What information must be reported on gmp 147 - virginia?
Businesses must report total gross receipts for the tax year, including any exemptions or deductions allowed.
How can I manage my gmp 147 - virginia directly from Gmail?
It's easy to use pdfFiller's Gmail add-on to make and edit your gmp 147 - virginia and any other documents you get right in your email. You can also eSign them. Take a look at the Google Workspace Marketplace and get pdfFiller for Gmail. Get rid of the time-consuming steps and easily manage your documents and eSignatures with the help of an app.
How can I send gmp 147 - virginia to be eSigned by others?
When your gmp 147 - virginia is finished, send it to recipients securely and gather eSignatures with pdfFiller. You may email, text, fax, mail, or notarize a PDF straight from your account. Create an account today to test it.
How do I complete gmp 147 - virginia online?
Easy online gmp 147 - virginia completion using pdfFiller. Also, it allows you to legally eSign your form and change original PDF material. Create a free account and manage documents online.
Fill out your gmp 147 - virginia online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Gmp 147 - Virginia is not the form you're looking for?Search for another form here.
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.