Get the free GMP CHECKLIST
Show details
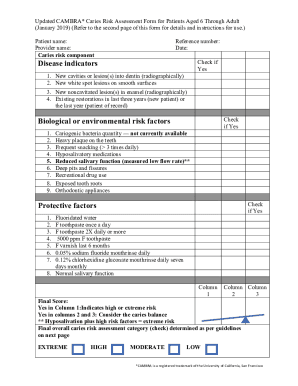
Batch Processing Records BPR Whether BPR are based on current How BPR are designed to avoid transcription errors. Page 7 Quality Control Area Whether QC area is independent of production area. Whether QC carries out its own physico-chemical testing biological testing microbiological testing sterility testing and Instrumental testing. Are there general change rooms in Are toilets change room separate from mfg. Area Pls specify number of washing station toilets provided for number of users....
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign gmp checklist

Edit your gmp checklist form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your gmp checklist form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing gmp checklist online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in to account. Start Free Trial and sign up a profile if you don't have one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit gmp checklist. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Create an account to find out for yourself how it works!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out gmp checklist

How to fill out a GMP checklist?
01
Review the checklist: Start by carefully examining the GMP checklist to understand its requirements. Familiarize yourself with the sections, categories, and specific items that need to be addressed.
02
Gather necessary documentation: Collect all relevant documents, records, and data that pertain to the GMP checklist items. This may include standard operating procedures (SOPs), batch records, calibration records, and training logs, among others.
03
Evaluate compliance: Go through each item on the checklist and assess if your organization meets the required standards. Determine if there are any gaps or areas that need improvement. Document any non-compliances observed.
04
Document actions taken: Throughout the checklist, record the actions you have taken to comply with each item. This could include providing references to specific documents, detailing corrective actions, or highlighting any preventive measures implemented.
05
Assign responsibilities: Identify the individuals or departments responsible for implementing and maintaining compliance with each checklist item. Make sure to clearly document who is accountable for each requirement.
06
Review and sign off: Once you have completed filling out the GMP checklist, review it again to ensure accuracy and completeness. Obtain the appropriate signatures or approvals as necessary, indicating that the checklist has been thoroughly evaluated and addressed.
Who needs a GMP checklist?
01
Pharmaceutical companies: GMP checklists are essential for pharmaceutical manufacturers to ensure that their products are produced in a safe and controlled environment, meeting the quality standards required by regulatory agencies.
02
Food and beverage industry: Food and beverage companies also require GMP checklists to maintain product integrity and safety. These checklists help ensure that industry regulations and guidelines are followed throughout the production process.
03
Medical device manufacturers: GMP checklists are vital for medical device manufacturers to ensure their products are safe, effective, and meet the necessary quality standards. They help in maintaining consistency and adherence to regulatory requirements.
04
Contract manufacturers: Businesses that outsource manufacturing processes need GMP checklists to monitor and verify that their contracted partners are adhering to established quality standards and regulations.
05
Regulatory authorities: GMP checklists are used by regulatory authorities to assess compliance during inspections and audits of manufacturing facilities. These checklists serve as a reference to verify if the organizations are meeting regulatory requirements.
In summary, filling out a GMP checklist involves reviewing the checklist, gathering documentation, evaluating compliance, documenting actions taken, assigning responsibilities, and reviewing and signing off on the checklist. GMP checklists are necessary for pharmaceutical companies, food and beverage industry, medical device manufacturers, contract manufacturers, and regulatory authorities.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make edits in gmp checklist without leaving Chrome?
Install the pdfFiller Chrome Extension to modify, fill out, and eSign your gmp checklist, which you can access right from a Google search page. Fillable documents without leaving Chrome on any internet-connected device.
Can I sign the gmp checklist electronically in Chrome?
As a PDF editor and form builder, pdfFiller has a lot of features. It also has a powerful e-signature tool that you can add to your Chrome browser. With our extension, you can type, draw, or take a picture of your signature with your webcam to make your legally-binding eSignature. Choose how you want to sign your gmp checklist and you'll be done in minutes.
How do I complete gmp checklist on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your gmp checklist from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
What is gmp checklist?
GMP checklist stands for Good Manufacturing Practices checklist. It is a tool used to ensure that products are consistently produced and controlled according to quality standards.
Who is required to file gmp checklist?
Companies involved in the manufacturing of products are required to file GMP checklist.
How to fill out gmp checklist?
The GMP checklist should be filled out by documenting all steps of the manufacturing process and ensuring that each step meets quality standards.
What is the purpose of gmp checklist?
The purpose of the GMP checklist is to maintain quality standards in manufacturing processes.
What information must be reported on gmp checklist?
Information such as production processes, quality control measures, equipment maintenance records, and employee training records must be reported on the GMP checklist.
Fill out your gmp checklist online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Gmp Checklist is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.