Get the free PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE IS NOW AVAILABLE FROM VFC - ...
Show details
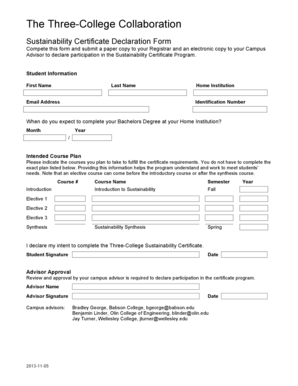
This document provides information to California Vaccines for Children Program Providers regarding the availability, administration, and recommendations for the new rotavirus vaccine, RotaTeq™,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign pentavalent bovine-human reassortant rotavirus

Edit your pentavalent bovine-human reassortant rotavirus form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your pentavalent bovine-human reassortant rotavirus form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit pentavalent bovine-human reassortant rotavirus online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in to your account. Click Start Free Trial and sign up a profile if you don't have one yet.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit pentavalent bovine-human reassortant rotavirus. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, it's always easy to work with documents. Try it!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out pentavalent bovine-human reassortant rotavirus

How to fill out PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE IS NOW AVAILABLE FROM VFC
01
Obtain the PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE from your local VFC provider.
02
Ensure the vaccine is stored at the appropriate temperature before administration.
03
Review the patient’s vaccination history to confirm eligibility for this vaccine.
04
Prepare the vaccine according to the manufacturer's instructions.
05
Administer the vaccine to the eligible patient following standard vaccination protocols.
06
Document the vaccination in the patient's medical record.
07
Provide any necessary information to the patient’s guardian about possible side effects and follow-up.
Who needs PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE IS NOW AVAILABLE FROM VFC?
01
Infants and young children who are at risk of rotavirus infection.
02
Children under 5 years of age to prevent severe gastroenteritis caused by rotavirus.
03
Children who are receiving routine immunizations through the VFC program.
Fill
form
: Try Risk Free






People Also Ask about
What are the 4 types of rotavirus vaccines?
Types Rotarix. Rotarix vaccine for oral administration. Rotateq. Rotateq is a live, oral pentavalent vaccine that contains five rotavirus strains produced by reassortment. Rotavac. Rotavac was licensed for use in India in 2014 and is manufactured by Bharat Biotech International Limited. Rotavin-M1. Lanzhou lamb. Rotasiil.
Is the rotavirus vaccine available in the US?
CDC recommends that infants get rotavirus vaccine to protect against rotavirus disease. Two rotavirus vaccines are currently licensed for use in infants in the United States. RotaTeq® (RV5) has been approved for use since 2006, and is given in three doses at 2 months, 4 months, and 6 months of age.
Can humans get bovine rotavirus?
Bovine G6 strains were reported in many countries to be associated with P[1], P[5], and P[11] (11, 12, 14–16). Two G8P[1] strains, NIC522 and B12, were described as evidence of direct transmission of bovine rotaviruses to humans (18, 19).
What is the pentavalent rotavirus vaccine?
This vaccine is used to help prevent a certain virus infection (rotavirus) in infants and young children. The infection can cause fever, vomiting, and diarrhea, which can lead to severe (rarely fatal) dehydration. Vaccination is the best way to protect against this infection.
What is the pentavalent human bovine reassortant rotavirus vaccine?
Pentavalent human-bovine rotavirus vaccine (HBRV) is an oral vaccine containing five live attenuated human-bovine reassortant strains containing the human serotypes responsible for the majority of rotavirus cases worldwide.
What is a pentavalent rotavirus vaccine?
Indications and Usage for RotaTeq® (Rotavirus Vaccine, Live, Oral, Pentavalent) RotaTeq is indicated for the prevention of rotavirus gastroenteritis in infants and children caused by Types G1, G2, G3, G4, and G9 when administered as a 3-dose series to infants between the ages of 6 to 32 weeks.
What is the controversy with the rotavirus vaccine?
Some studies suggest that rotavirus vaccination possibly causes a small increase in the risk of intussusception, a type of blockage.
What is the new pentavalent rotavirus vaccine?
Indications and Usage for RotaTeq® (Rotavirus Vaccine, Live, Oral, Pentavalent) RotaTeq is indicated for the prevention of rotavirus gastroenteritis in infants and children caused by Types G1, G2, G3, G4, and G9 when administered as a 3-dose series to infants between the ages of 6 to 32 weeks.
What is the controversy with the rotavirus vaccine?
Some studies suggest that rotavirus vaccination possibly causes a small increase in the risk of intussusception, a type of blockage.
What is the current rotavirus vaccine?
RotaTeq® (RV5) has been approved for use since 2006, and is given in three doses at 2 months, 4 months, and 6 months of age. Rotarix® (RV1) has been approved for use since 2008, and is given in two doses at 2 months and 4 months of age.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE IS NOW AVAILABLE FROM VFC?
PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE is a vaccine designed to protect children from severe rotavirus gastroenteritis. It is now available through the Vaccines for Children (VFC) program to ensure that eligible children receive this important vaccination at no cost.
Who is required to file PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE IS NOW AVAILABLE FROM VFC?
Healthcare providers who administer the PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE are required to file necessary documentation with the VFC program to record vaccine administration and inventory management.
How to fill out PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE IS NOW AVAILABLE FROM VFC?
To fill out the documentation for the PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE, healthcare providers should accurately record patient information, vaccination date, vaccine lot number, and any reactions or follow-ups necessary as per VFC guidelines.
What is the purpose of PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE IS NOW AVAILABLE FROM VFC?
The purpose of the PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE is to provide immunity against rotavirus, which can cause severe diarrhea, dehydration, and even hospitalization in young children. The VFC makes it accessible to those in need.
What information must be reported on PENTAVALENT, BOVINE-HUMAN REASSORTANT ROTAVIRUS VACCINE IS NOW AVAILABLE FROM VFC?
Providers must report information including patient demographic details, vaccination date, vaccine manufacturer and lot number, and any adverse events following vaccination as required by the VFC program policies.
Fill out your pentavalent bovine-human reassortant rotavirus online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Pentavalent Bovine-Human Reassortant Rotavirus is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.