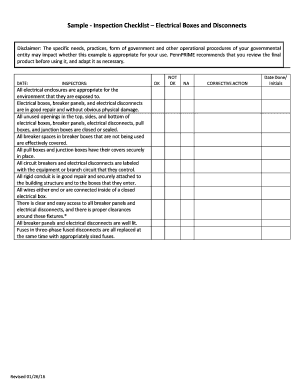
Get the free Laboratory Close-Out Procedure Principal Investigator: Department ... - ehs unc
Show details
Provide thorough laboratory deactivation to assure the safety of the space in accordance with all applicable EPA, OSHA, NIH, CDC, and state regulations after laboratories are relocated, renovated,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign laboratory close-out procedure principal

Edit your laboratory close-out procedure principal form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your laboratory close-out procedure principal form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit laboratory close-out procedure principal online
Follow the guidelines below to benefit from a competent PDF editor:
1
Check your account. In case you're new, it's time to start your free trial.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit laboratory close-out procedure principal. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out laboratory close-out procedure principal

How to fill out laboratory close-out procedure principal:
01
Start by reviewing the specific guidelines and requirements of the laboratory close-out procedure principal. Familiarize yourself with any standard protocols or templates provided.
02
Gather all necessary information and documentation related to the laboratory close-out, including any experimental data, equipment records, maintenance logs, and safety protocols.
03
Follow the designated format or template provided for the laboratory close-out procedure principal. Ensure that all relevant sections and fields are completed accurately and comprehensively.
04
Document any deviations or discrepancies encountered during the close-out process. Be sure to provide explanations or corrective actions taken to address these issues.
05
Include a comprehensive inventory of all laboratory equipment, chemicals, and materials used in the experiment. Note any disposal or transfer procedures implemented for these items.
06
Validate and verify the accuracy of all data and records included in the laboratory close-out procedure principal. Perform any necessary cross-referencing or quality checks.
07
Ensure that all required signatures and approvals are obtained before finalizing the laboratory close-out procedure principal.
08
Once completed, store the laboratory close-out procedure principal in a secure location and make it accessible to appropriate personnel for future reference or auditing purposes.
Who needs laboratory close-out procedure principal:
01
Laboratory managers or supervisors who are responsible for overseeing and coordinating the closure of laboratory operations.
02
Researchers or scientists involved in laboratory experiments or activities that require proper close-out procedures to ensure the safe and accurate conclusion of their work.
03
Regulatory authorities or auditors who may require proof of adherence to laboratory close-out protocols and guidelines.
04
Institutions or organizations that have specific policies or standard operating procedures in place for laboratory operations, including the closure process.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify laboratory close-out procedure principal without leaving Google Drive?
You can quickly improve your document management and form preparation by integrating pdfFiller with Google Docs so that you can create, edit and sign documents directly from your Google Drive. The add-on enables you to transform your laboratory close-out procedure principal into a dynamic fillable form that you can manage and eSign from any internet-connected device.
How do I make changes in laboratory close-out procedure principal?
pdfFiller not only allows you to edit the content of your files but fully rearrange them by changing the number and sequence of pages. Upload your laboratory close-out procedure principal to the editor and make any required adjustments in a couple of clicks. The editor enables you to blackout, type, and erase text in PDFs, add images, sticky notes and text boxes, and much more.
Can I create an electronic signature for signing my laboratory close-out procedure principal in Gmail?
Upload, type, or draw a signature in Gmail with the help of pdfFiller’s add-on. pdfFiller enables you to eSign your laboratory close-out procedure principal and other documents right in your inbox. Register your account in order to save signed documents and your personal signatures.
What is laboratory close-out procedure principal?
The laboratory close-out procedure principal is a set of guidelines and protocols that outline the steps and requirements for properly closing out a laboratory facility or project.
Who is required to file laboratory close-out procedure principal?
The laboratory close-out procedure principal is typically filed by the responsible parties or individuals overseeing the laboratory facility or project, such as the project manager or laboratory supervisor.
How to fill out laboratory close-out procedure principal?
The laboratory close-out procedure principal should be filled out by following the specific instructions and requirements outlined in the document. It may involve providing information such as the project details, equipment disposal methods, data management plans, and personnel responsibilities.
What is the purpose of laboratory close-out procedure principal?
The purpose of the laboratory close-out procedure principal is to ensure that proper protocols are followed when closing out a laboratory facility or project. It helps to ensure the safe disposal of hazardous materials, proper documentation of project activities, and adherence to regulatory requirements.
What information must be reported on laboratory close-out procedure principal?
The exact information to be reported on the laboratory close-out procedure principal may vary depending on the specific requirements of the project or facility. However, it may include details on equipment inventory, disposal plans for hazardous materials, data management and storage plans, and personnel responsibilities.
Fill out your laboratory close-out procedure principal online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Laboratory Close-Out Procedure Principal is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.