Get the free ANVISA DIRECTIVE- RDC No
Show details
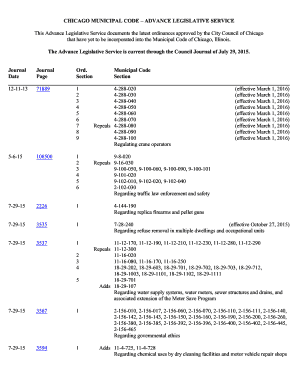
1 ANV ISA DIRECTIVE RDC No. 7, 10 FEBRUARY 2015 (COSMETICS & TOILETRIES AND PERFUMES REGISTRATION PROCEDURES AND REQUIREMENTS) The technical requirements are regulated for the regularization of toiletries,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign anvisa directive- rdc no

Edit your anvisa directive- rdc no form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your anvisa directive- rdc no form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit anvisa directive- rdc no online
Follow the guidelines below to benefit from a competent PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit anvisa directive- rdc no. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out anvisa directive- rdc no

How to Fill out ANVISA directive- RDC No.:
01
Start by gathering all the necessary information and documents required for filling out the ANVISA directive- RDC No. This may include the directive itself, any supporting documents, and relevant information about the product or procedure.
02
Carefully read through the directive and ensure you understand all the requirements and guidelines outlined. Take note of any specific instructions, deadlines, or additional forms that need to be filled out.
03
Begin filling out the necessary forms or documents according to the instructions provided. Pay close attention to details such as correct spelling, accurate information, and complete responses. Double-check your work for any errors or omissions.
04
If any additional supporting documents are required, make sure to include them in the appropriate sections or attachments. These documents may include certificates, test reports, labeling information, or any other relevant paperwork that is specified in the ANVISA directive.
05
Follow any specific formatting or submission guidelines stated in the directive. This may include using specific templates or submitting the forms electronically through a designated online platform. Make sure to comply with all the formatting requirements to avoid any delays or rejections.
Who needs ANVISA directive- RDC No.:
01
Any individual or organization involved in the fields of healthcare, pharmaceuticals, medical devices, cosmetics, food products, or any other industry regulated by ANVISA (Agência Nacional de Vigilância Sanitária) may need to comply with ANVISA directive RDC No.
02
Manufacturers or importers of medical products, drugs, food supplements, cosmetics, or any other goods that require ANVISA's authorization or approval may be required to fill out and submit the ANVISA directive- RDC No.
03
Healthcare professionals, researchers, or institutions conducting clinical trials, experiments, or studies that involve the use of drugs, medical devices, or any regulated product may also need to adhere to the ANVISA directive- RDC No.
In summary, anyone involved in industries regulated by ANVISA, specifically those requiring authorization, approval, or conducting studies with regulated products, may need to fill out and comply with ANVISA directive- RDC No.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is anvisa directive- rdc no?
The Anvisa Directive - RDC No is a regulation issued by the Brazilian Health Regulatory Agency (Anvisa) that establishes requirements and procedures related to health products.
Who is required to file anvisa directive- rdc no?
Manufacturers, importers, and distributors of health products are required to file Anvisa Directive - RDC No.
How to fill out anvisa directive- rdc no?
To fill out Anvisa Directive - RDC No, companies must follow the guidelines and requirements outlined in the regulation and submit the necessary documentation to Anvisa.
What is the purpose of anvisa directive- rdc no?
The purpose of Anvisa Directive - RDC No is to regulate the registration, manufacturing, import, distribution, and monitoring of health products in Brazil.
What information must be reported on anvisa directive- rdc no?
The information that must be reported on Anvisa Directive - RDC No includes details about the product, manufacturing processes, quality control measures, and compliance with regulations.
How can I send anvisa directive- rdc no to be eSigned by others?
When you're ready to share your anvisa directive- rdc no, you can send it to other people and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail. You can also notarize your PDF on the web. You don't have to leave your account to do this.
How do I make edits in anvisa directive- rdc no without leaving Chrome?
Download and install the pdfFiller Google Chrome Extension to your browser to edit, fill out, and eSign your anvisa directive- rdc no, which you can open in the editor with a single click from a Google search page. Fillable documents may be executed from any internet-connected device without leaving Chrome.
Can I create an eSignature for the anvisa directive- rdc no in Gmail?
Use pdfFiller's Gmail add-on to upload, type, or draw a signature. Your anvisa directive- rdc no and other papers may be signed using pdfFiller. Register for a free account to preserve signed papers and signatures.
Fill out your anvisa directive- rdc no online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Anvisa Directive- Rdc No is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.