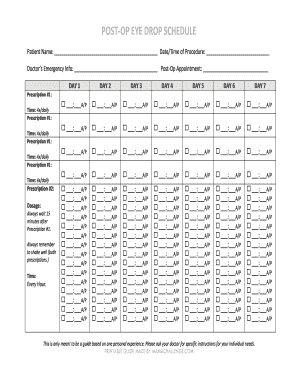
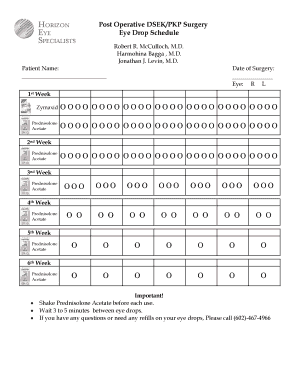
UK The Practice Group Eye Drops: Compliance Sheet 2015-2025 free printable template
Show details
5 May 2015 ... Only the number of drops being administered to the eye and the ... The EDC appears to be a cost-effective way at improving patients' use of topical ocular medications. Keywords: glaucoma,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign eye drop schedule template form

Edit your printable eye drop chart form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your eye drop chart after cataract surgery form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit eye drop chart online
In order to make advantage of the professional PDF editor, follow these steps:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit cataract eye drop chart form. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
It's easier to work with documents with pdfFiller than you could have believed. You may try it out for yourself by signing up for an account.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out eye drop chart for cataract surgery form

How to fill out UK The Practice Group Eye Drops: Compliance Sheet
01
Gather all necessary information before starting the compliance sheet.
02
Open the compliance sheet document provided by The Practice Group.
03
Fill in the date on which the eye drops were administered.
04
Record the patient's name and details accurately.
05
Specify the type of eye drops given to the patient.
06
Document the dosage of the eye drops administered.
07
Note the time of administration and any specific instructions followed.
08
Include any observations or comments regarding the patient's response.
09
Validate all information entered and ensure it is accurate.
10
Save the completed compliance sheet and keep it in the patient's records.
Who needs UK The Practice Group Eye Drops: Compliance Sheet?
01
Healthcare professionals administering eye drops.
02
Clinics or hospitals offering ophthalmic treatments.
03
Patients receiving treatment with The Practice Group Eye Drops.
04
Regulatory bodies monitoring compliance in healthcare settings.
Fill
eye drops schedule
: Try Risk Free






People Also Ask about cataract surgery eye drops schedule chart
How many hours apart is 3 times a day for eye drops?
For ophthalmic dosage form (eye drops): For glaucoma or ocular hypertension: Adults and children 2 years of age and older—One drop in the affected eye 3 times a day, about 8 hours apart.
What are the 3 drops for after cataract surgery?
There are 3 types of eye drops that support eye health after cataract surgery: Antibiotic eye drops. Anti-inflammatory eye drops. Lubricating eye drops.
How many days does a 2.5 ml bottle of Latanoprost last?
In investigating efficacy of PGA drop instillation, Fiscella et al reported the SD of branded latanoprost as roughly 5 drops per bottle. Since glaucoma is a bilateral disease and patients apply PGA drops once a day to both eyes, on average, a 2.5 ml size of PGA can last for 45 days (8.1 bottles of medication per year).
How many hours apart should you take antibiotics 3 times a day?
It is usually taken every 12 hours (twice a day) or every 8 hours (three times a day) with or without food. The length of your treatment depends on the type of infection that you have.
How many doses are in a 2.5 ml bottle of Latanoprost?
Each dropper container contains 2.5 ml eye drops solution corresponding to approximately 80 drops of solution.
Does 3 times a day mean every 8 hours?
When the medicine label on the medicine says to take three times day it generally means 'take every 8 hours'. For example we divide 8 hours into 24 hours which gives us 3. Therefore four times a day would be 'every 6 hours,' (24 divided by 6 = 4).
What time should I take meds 3 times a day?
A medicine written three times daily has enough leeway in its dosing regimen that it may be taken morning/noon/night at the patient's convenience. In these cases, taking the medication an hour or two earlier or later will not affect the medication's effectiveness.
What is three times a day in medical terms?
ter die sumendum three times a day.
Should you blink after eye drops?
Don't Blink After applying eyedrops, many people believe they should blink a lot to spread the drops around the eye. But this is ineffective. Blinking may cause some of the drop to leak out, hindering optimal absorption.
Can I put eye drops 3 times a day?
Eye drops can be used multiple times a day, depending on your symptoms.
How many hours apart are eye drops?
Remember: Try to put the drop in at the same time each day. If your drop needs to be put in twice a day, try to leave 12 hour gaps between drops. If you have to use more than one eye drop, wait at least five minutes between them (or longer if advised by your ophthalmologist).
How long should 2.5 ml eye drops last?
If you use drops in both eyes once a day: A 2.5 ml bottle should last about a month. A 5.0 ml bottle should last approximately 60 days. A 7.5 ml bottle may last 90 days.
How long do you have to put drops in your eyes after cataract surgery?
Generally, you'll be provided with enough eye drops to last you for four weeks, and should continue using them until you run out. If you run out before the four weeks is up, then your GP will usually provide you with additional eye drops.
How long should eye drops last?
How long can you use eye drops after opening them? Many manufacturers recommend that you throw away eye drops 28 days after opening the bottle. This is because the preservatives inside can start to break down and allow bacteria to grow.
Which eye drops first after cataract surgery?
The following drops should be applied to your operated eye only, starting the morning after surgery. Chloramphenicol One drop, four times Antibiotic (store in fridge) a day for 2 weeks, then stop. Dexamethasone One drop, four times Anti- (Maxidex) a day for 4 weeks, inflammatory then stop.
What is the schedule for eye drops after cataract surgery?
The recommended drop schedule after cataract surgery is 1 drop twice a day in the operative eye for a minimum of three weeks. The Preservative Free Artificial Tears can be used up to 4 times daily or as needed for comfort. With steroid eye drops, doses need to be spread out a few hours.
How long should you wait between eye drops?
Abstract. Purpose: Patients are usually advised to wait 5 minutes between eye drops. This delay supposedly allows the first drop not to be washed out by the second one, thereby increasing the combined effect.
How do you take eye drops 3 times a day?
For drops that are required twice a day, the ideal dosing regimen is every 12 hours, and for drops that are dosed three times a day, the ideal dosing regimen is every 8 hours. However, sometimes that is not practical, and wiggle room of an hour or two (early or late) should be fine.
How many drops are in a 2.5 ml bottle of eye drops?
For 2.5 mL bottles, the mean number of drops ranged from 75.3–101.7 and 72–102.3 in the vertical and horizontal orientations, respectively. For 5 mL bottles, the range was 111–209.3 and 115–189 drops in the vertical and horizontal orientations, respectively.
How long should prednisolone eye drops be used after cataract surgery?
Steroids: Typically, we recommend Prednisolone 4 times daily for the first 2 weeks following routine cataract surgery, then cut back to 2 times daily for the next 2 weeks, then discontinue. Artificial Tears: Immediately following cataract surgery, patients may resume using artificial tears.
Our user reviews speak for themselves
Read more or give pdfFiller a try to experience the benefits for yourself
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send printable eye drop medication chart for eSignature?
When your printable cataract surgery eye is finished, send it to recipients securely and gather eSignatures with pdfFiller. You may email, text, fax, mail, or notarize a PDF straight from your account. Create an account today to test it.
Can I create an electronic signature for the printable cataract surgery eye in Chrome?
You can. With pdfFiller, you get a strong e-signature solution built right into your Chrome browser. Using our addon, you may produce a legally enforceable eSignature by typing, sketching, or photographing it. Choose your preferred method and eSign in minutes.
How do I edit printable cataract surgery eye straight from my smartphone?
You can do so easily with pdfFiller’s applications for iOS and Android devices, which can be found at the Apple Store and Google Play Store, respectively. Alternatively, you can get the app on our web page: https://edit-pdf-ios-android.pdffiller.com/. Install the application, log in, and start editing printable cataract surgery eye right away.
What is UK The Practice Group Eye Drops: Compliance Sheet?
UK The Practice Group Eye Drops: Compliance Sheet is a regulatory document that ensures compliance with safety and quality standards for the manufacturing and distribution of eye drop products in the UK.
Who is required to file UK The Practice Group Eye Drops: Compliance Sheet?
Manufacturers, distributors, and other stakeholders involved in the production and sale of eye drops in the UK are required to file the Compliance Sheet.
How to fill out UK The Practice Group Eye Drops: Compliance Sheet?
To fill out the Compliance Sheet, stakeholders must provide specific product information, safety data, manufacturing processes, and quality control measures in the designated sections of the document.
What is the purpose of UK The Practice Group Eye Drops: Compliance Sheet?
The purpose of the Compliance Sheet is to ensure that eye drop products meet regulatory standards for safety, efficacy, and quality, thereby protecting public health.
What information must be reported on UK The Practice Group Eye Drops: Compliance Sheet?
The Compliance Sheet must report information such as product name, batch number, ingredients, manufacturing date, expiry date, safety assessments, and any relevant test results.
Fill out your printable cataract surgery eye online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Printable Cataract Surgery Eye is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.