Comprehensive Guide on IBD Clinical Trials Referral Form
Understanding inflammatory bowel disease (IBD)
Inflammatory Bowel Disease (IBD) refers to a group of chronic inflammatory conditions of the gastrointestinal tract, primarily including Crohn's disease and ulcerative colitis. These diseases significantly impact the lives of individuals due to their debilitating symptoms, which can range from persistent diarrhea and abdominal pain to fatigue and weight loss. Diagnosis typically involves a combination of medical history evaluation, endoscopic procedures, imaging tests, and laboratory analyses. Understanding IBD's nuanced characteristics and the ways it affects patients is vital for exploring potential treatment options, including participating in clinical trials.
Clinical trials serve as a crucial component in the progression of IBD treatments. These trials explore new therapeutic interventions, assess their safety and efficacy, and ultimately help to improve patient outcomes. By participating in clinical trials, patients gain access to cutting-edge treatments that may not yet be widely available and contribute to the broader understanding of IBD management.
Navigating the clinical trials landscape
Clinical trials are carefully designed research studies conducted to evaluate new medical interventions. They can be divided into various types based on their objectives, such as treatment trials, prevention trials, diagnostic trials, and quality of life trials. Each of these categories contributes to the extensive knowledge base concerning IBD and its management.
Treatment trials focus on new therapies aimed at alleviating symptoms and improving quality of life for patients.
Prevention trials assess strategies designed to prevent the onset of IBD in at-risk populations.
Diagnostic trials investigate new methods for diagnosing IBD earlier and more accurately.
Quality of life trials examine the impacts of IBD on daily life and potential interventions to enhance patient well-being.
Participating in clinical trials offers numerous benefits for IBD patients. Firstly, patients may gain access to groundbreaking treatments not yet available to the public, ultimately advancing their medical care. Moreover, clinical trial participants receive close monitoring by healthcare professionals, ensuring a higher standard of care and support throughout their treatment journey.
Overview of the IBD clinical trials referral form
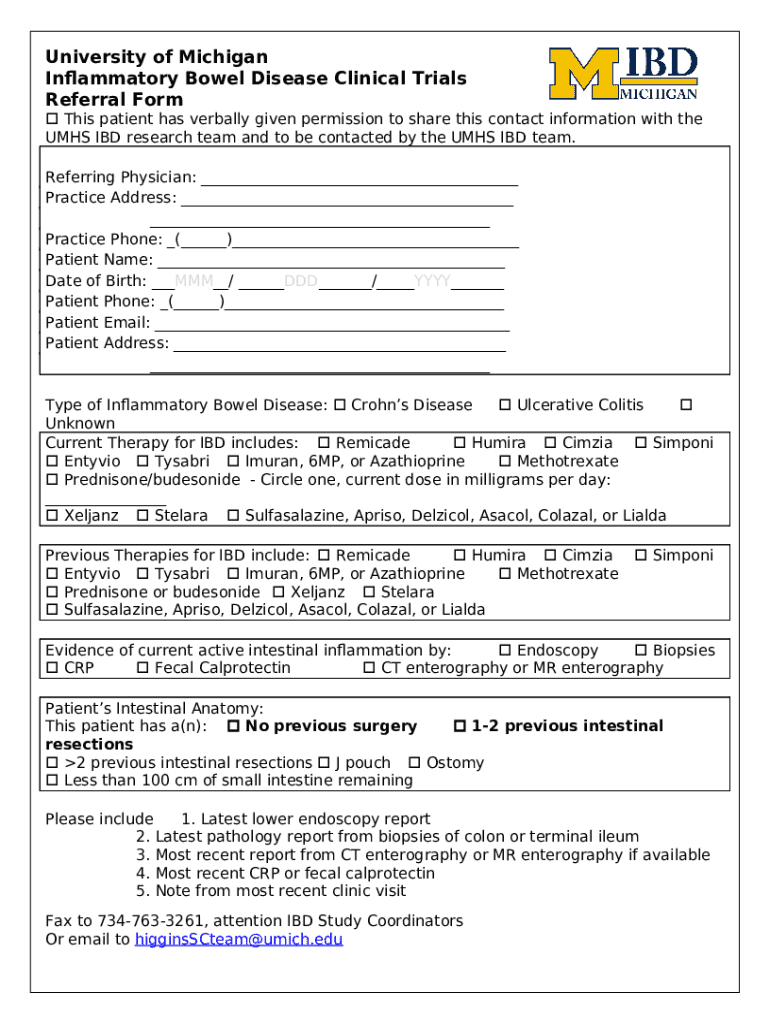
The IBD clinical trials referral form is a critical document that healthcare providers use to refer patients for potential participation in clinical trials tailored to inflammatory bowel disease. This form not only streamlines the referral process but also ensures that all relevant patient information is captured efficiently. Healthcare providers can ascertain a patient's fitness for trial participation based on the data included in this form.
The referral form should be utilized when a patient expresses interest in participating in clinical trials or when a healthcare provider identifies a potentially suitable trial for their patient. Typically, any licensed healthcare professional, including gastroenterologists, primary care physicians, and nurse practitioners, can refer patients using the IBD clinical trials referral form.
Step-by-step guide to filling out the IBD clinical trials referral form
Completing the IBD clinical trials referral form requires careful attention to detail to accurately represent the patient's health status and medical history. The required information typically includes:
This includes the patient's name, contact information, date of birth, and insurance details.
Providers need to detail the patient's IBD diagnosis, any concurrent conditions, and relevant family history.
This section should detail the treatments the patient has undergone, their effectiveness, and any adverse reactions experienced.
Additionally, supporting documents such as necessary medical records and insurance information will facilitate the referral process. Completing the form accurately and providing ample context about the patient's health status can significantly enhance the likelihood of a successful referral.
Editing and customizing your referral form
To effectively manage the IBD clinical trials referral form, utilizing tools like pdfFiller can significantly enhance the experience of both healthcare providers and patients. pdfFiller allows users to easily edit and customize the referral form, ensuring that all necessary information is captured accurately.
One notable feature of pdfFiller is the ability to incorporate electronic signatures directly into the document. This streamlines the process, eliminating the delays typically associated with physical signatures. Healthcare professionals can also collaborate seamlessly, sharing the form with colleagues for feedback or additional input, thus enhancing the overall quality of the referral.
Submitting the IBD clinical trials referral form
When it comes to submitting the IBD clinical trials referral form, several options exist, depending on the specifications of the clinical trial and institutional policies. Forms can often be sent electronically via email or uploaded directly to the clinical trials database, ensuring a timely response.
Understanding the response timeframes associated with the submission process is essential. Typically, once the referral form is submitted, there is a timeframe within which the clinical trial team will evaluate the application and communicate with the referring healthcare provider regarding the next steps. After submission, establishing follow-up procedures can help ensure that both the healthcare provider and patient remain informed throughout the process.
Connecting with clinical trials
Identifying current clinical trials suitable for IBD patients can be done through multiple resources. Numerous online databases and registries offer search functionalities for locating active IBD clinical trials based on patient criteria, geographical location, and treatment goals.
ClinicalTrials.gov provides a comprehensive registry of trials, allowing users to narrow their results based on various filters.
The Crohn's & Colitis Foundation offers resources specifically for IBD patients looking to navigate available trials.
Local research institutions and hospitals often list available trials and can offer direct contact to trial coordinators for further details.
Understanding trial eligibility criteria is crucial for ensuring that patients meet necessary qualifications. This information not only directs discussions with healthcare teams but also empowers patients to make informed decisions about their treatment options.
Continuous communication and updates
Establishing a routine for regular follow-ups is essential for patients and providers participating in clinical trials. Maintaining open lines of communication ensures that any concerns are addressed promptly and that participants are kept informed of their trial progress.
Tracking clinical trial progress often involves direct communication with clinical trial teams. Patients are encouraged to provide feedback and report any changes in their health status, as healthcare providers rely on this information to make necessary adjustments to treatment plans.
Community and support resources
Connecting with others who understand the journey of living with IBD can greatly enhance a patient's experience. Joining IBD support groups, either locally or online, can provide not just emotional support but also valuable knowledge regarding coping strategies and clinical trial opportunities.
Online forums serve as spaces where IBD patients can discuss experiences, share insights, and receive encouragement from peers.
Educational resources and workshops organized by local chapters of IBD organizations can provide patients and families with essential information about managing their condition.
Utilizing these community and support resources not only empowers patients but also fosters an environment of understanding and collective advocacy for IBD issues.
Physician-to-physician consultations
In situations requiring specialized knowledge, physician-to-physician consultations can enhance patient care. The IBD clinical trials referral form plays a significant role in facilitating effective communication between referring and consulting healthcare professionals.
Utilizing the referral form allows for the seamless exchange of patient information, ensuring that all parties involved have a comprehensive understanding of the patient’s medical history and treatment goals. This direct line of communication can accelerate decision-making and improve the quality of care for IBD patients.
Nutrition and lifestyle considerations for IBD patients
Diet plays a crucial role in managing symptoms and improving the quality of life for those living with IBD. While individual dietary needs can vary widely among patients, many find that specific foods can trigger their symptoms, while others can help alleviate them.
This may involve identifying and avoiding trigger foods that exacerbate symptoms.
Incorporating well-balanced meals rich in fiber, vitamins, and minerals is often recommended to support overall health.
Staying hydrated is essential, especially during flare-ups when diarrhea can lead to significant fluid loss.
Consulting with a nutritionist familiar with IBD can enhance dietary choices and lifestyle adjustments tailored to individual symptoms, optimizing the management of their condition.
Advantages of using pdfFiller for document management
For healthcare professionals dealing with the IBD clinical trials referral form, pdfFiller offers a comprehensive solution for document management. Its cloud-based platform ensures accessibility from anywhere, which is particularly valuable in today’s mobile healthcare environment.
Security features provided by pdfFiller protect sensitive patient information, while collaborative functionalities enhance workflow efficiency among providers. By streamlining the management of referrals and other essential documents, pdfFiller empowers users to focus more on patient care and less on administrative burdens.