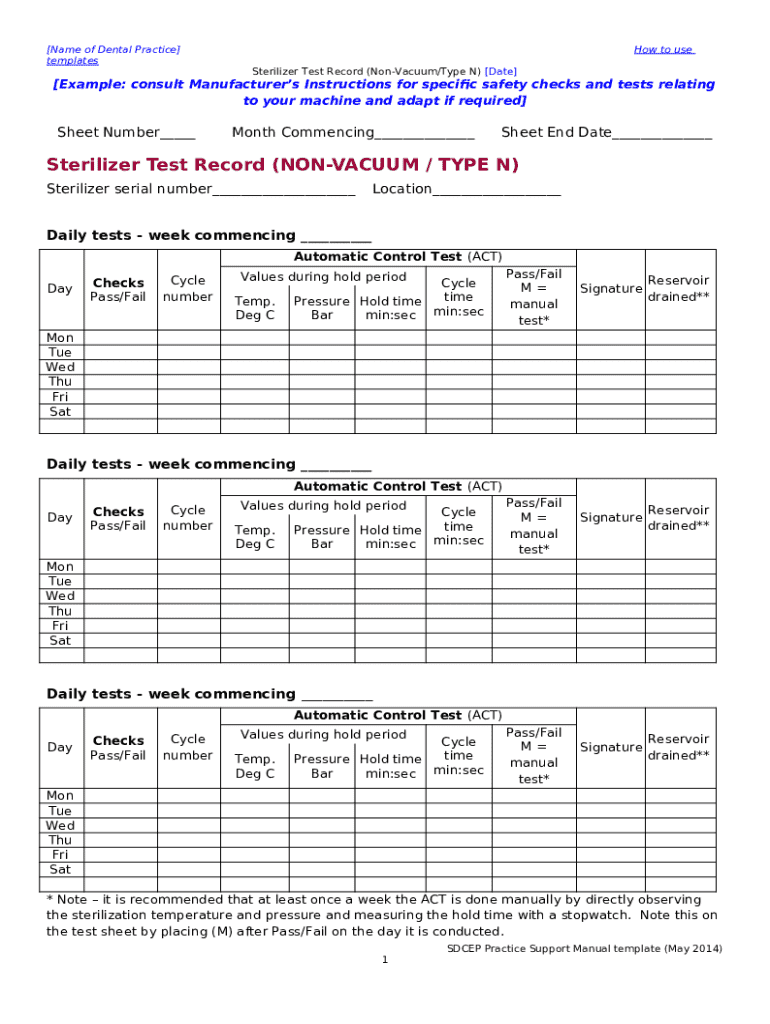
Comprehensive Guide to Non-Vacuum Type N Sterilizer Form
Understanding non-vacuum type N sterilizers
Non-vacuum type N sterilizers are designed to effectively eliminate microorganisms and pathogens without the need for a vacuum system. These sterilizers utilize steam to achieve sterilization, employing a gravity displacement cycle to drive steam into the sterilization chamber. This method is particularly popular in settings where simplicity and efficiency are paramount, such as dental offices and certain surgical environments.
The key characteristics of non-vacuum sterilization include the inability to remove air from the sterilization chamber prior to the steam process. This method relies on the natural tendency of steam to displace air, which means that sterilization may take longer for porous or hollow instruments compared to vacuum methods. Non-vacuum sterilizers are predominantly developed to address needs like speed, cost-effectiveness, and ease of use.
Historically, non-vacuum sterilizers have evolved significantly. Early models liberated steam through basic gravity techniques, often leading to less effective sterilization cycles. However, advancements in technology have enhanced their reliability and safety, making them a common choice in many medical and dental practices.
Working principles of non-vacuum type N sterilizers
The mechanism of action of non-vacuum type N sterilizers primarily revolves around the principles of steam sterilization. Upon closing the sterilization chamber, steam is introduced, displacing air. The temperature of the steam rises to at least 121°C (250°F), maintained for a specific duration to achieve effective sterilization. To ensure optimal results, the steam must contact all surfaces of the instruments to ensure the killing of resistant organisms.
Several parameters influence sterilization effectiveness, including exposure time, temperature, and the nature of the load being sterilized. Typically, a cycle will last around 30 to 60 minutes for items that do not require vacuum assistance. This makes it crucial to monitor and adjust cycles based on the sterilizer's specifications and load characteristics.
Maintaining correct steam temperature is critical for effective sterilization.
Sufficient time must be allowed for items to be thoroughly saturated with steam.
Proper loading of items ensures that all surfaces are effectively reached by steam.
The advantages of non-vacuum methods in certain applications include lower equipment costs, ease of operation, and reliability under controlled circumstances. They are particularly advantageous in facilities that sterilize unwrapped items or when rapid turnaround is required, enabling quick preparation between cases.
Comparing non-vacuum and vacuum-based sterilization
To understand the ins and outs of non-vacuum type N sterilizers, one must also consider the nuances of vacuum-based sterilization processes. Pre & post-vacuum autoclaves utilize a vacuum to remove air from the chamber prior to steam exposure. This process allows for more effective sterilization of porous items, making them crucial for certain applications, such as in surgical facilities handling a variety of instruments.
Key differences between gravity and pre & post-vacuum autoclaves lie in their operation and effectiveness. Vacuum systems perform better on complex instruments with hollow channels or intricate surfaces where air pockets can lead to sterilization failures. In contrast, non-vacuum methods are often simpler in design and operation but may face limitations concerning certain materials and configurations.
Non-vacuum systems often have fewer moving parts, reducing potential service issues.
Lower initial investment compared to vacuum systems can be appealing for smaller practices.
Non-vacuum methods may require longer cycles for effective sterilization of certain items.
Not suitable for all types of materials, particularly those that are porous or hollow.
Understanding these differences helps users make informed decisions about which type of sterilization process to implement, paving the way for effective sterilization practices tailored to specific environments.
Applications of non-vacuum type N sterilizers
Non-vacuum type N sterilizers find their niche across various environments and industries. They are especially well-suited for dental offices, outpatient surgery centers, and other medical facilities where quick turnover of non-porous instruments is essential. In these settings, the ability to execute rapid sterilization without complex operational procedures adds to their desirability.
Common instruments and materials sterilized using type N autoclaves include surgical instruments, dental tools, and non-woven textiles. Items that can withstand high temperatures and moisture are ideal candidates for this method. The flexibility in utility makes these sterilizers a foundational piece of equipment in many healthcare environments.
Tools used in dental procedures that require high levels of cleanliness.
Includes scalpels, scissors, and forceps that are used in operating rooms.
Sterilizing materials that can withstand the conditions inside an autoclave.
Case studies demonstrate the effective use of non-vacuum sterilizers in various healthcare operations. For instance, a dental practice successfully implemented a non-vacuum type N sterilizer to streamline its workflow, reducing instrument turnaround times significantly while ensuring high sterilization standards. Such practical examples highlight the importance of adapting sterilization practices to the specific needs of medical facilities.
Operational guidelines for maximizing efficiency
To operate a non-vacuum type N sterilizer effectively, it's essential to follow a step-by-step guide. Begin by ensuring the sterilization chamber is clean and free of any residual materials. Prepare the instruments by thoroughly cleaning and drying them to remove any contaminants. Next, load the chamber with care, ensuring items are not overcrowded, which can inhibit steam penetration.
Once loaded, close the door securely and select the appropriate cycle for your items. After the cycle concludes, allow the sterilized items to cool before unloading to avoid burns and ensure safety. Post-operation, maintain the sterilizer's cleanliness by conducting routine checks and cleaning protocols to maintain efficiency and prolong its lifespan.
Routine maintenance is key to extending the life of your sterilizer.
Ensure that items are arranged for optimal steam contact.
Keep track of time, temperature, and loading practices for consistent results.
Best practices for loading and unloading instruments can greatly influence sterilization outcomes. Prioritize larger items at the bottom, allowing smaller tools to fit neatly on top, leading to a more efficient steam pathway throughout the load.
Troubleshooting common issues with non-vacuum sterilizers
Identifying signs of sterilization failure can sometimes be subtle yet critical. Common indicators may include discoloration of items, retained moisture on instruments post-cycle, or inconsistent sterilization logs. When encountering these issues, it’s crucial to conduct a thorough evaluation to ascertain the root causes, which can range from improper loading to malfunctions within the sterilizer itself.
Common problems and their solutions vary but typically include errors in cycle selection, improper door sealing, or issues with steam generation. Regularly validating the functionality of the sterilizer and following routine maintenance protocols can significantly reduce the likelihood of these problems occurring.
Ensure correct settings are used for loading and item types.
Inspect seals to prevent steam leaks, a common cause of sterilization failures.
Engage with technicians periodically for comprehensive assessments.
When professional maintenance is required, it’s vital to document all previous cycles and issues encountered, which helps technicians diagnose the problems effectively. Keeping a maintenance log not only helps with compliance but also ensures that the sterilizer functions optimally over its lifespan.
Regulatory compliance and standards for sterilizers
Compliance with industry regulations is essential for any facility utilizing non-vacuum type N sterilizers. Regulatory bodies, including the FDA, have established guidelines pertaining to equipment performance, ensuring user and patient safety. Understanding these regulations plays a fundamental role in ensuring that sterilization practices align with safety standards.
The importance of compliance for safety and efficacy cannot be overstated. Sterilizers must undergo regular performance validation and adhere to defined standards, including those set by the Association for the Advancement of Medical Instrumentation (AAMI). Developing a robust compliance strategy safeguards facilities from potential liabilities and ensures patient safety.
Understand the requirements around sterilization equipment safety.
Ensure adherence to established protocols for sterilization methods.
Maintain detailed logs for operational transparency and inspection readiness.
Navigating certification processes for type N autoclaves can be complex. Facilities must stay informed about changes in regulations and ensure appropriate training for staff to uphold compliance continuously. This ongoing education is paramount for sustaining the integrity and safety of sterilization processes.
Calculating the cost of ownership for non-vacuum sterilizers
Understanding the total cost of ownership for non-vacuum type N sterilizers involves several factors, including initial purchase price, maintenance costs, and operational expenditures. These sterilizers typically offer a lower upfront cost compared to vacuum systems, making them accessible for smaller facilities. However, it's essential to account for their longevity and efficiency over time.
The initial investment versus long-term operational costs reveals a balanced consideration when evaluating options. While upfront purchases are crucial, ongoing maintenance and consumable supplies like steam indicators must be factored into the overall budget. Facilities should analyze anticipated usage levels to gauge overall cost-effectiveness effectively.
Analyze the upfront costs when selecting the sterilizer.
Plan for regular service checks and parts replacement.
Consider the costs of energy and consumables associated with sterilization.
A comprehensive cost-effectiveness analysis with other sterilization types can help in decision-making. Evaluating total costs in light of sterilization needs ensures that the chosen sterilization method aligns with both financial and operational goals.
FAQs about non-vacuum type N sterilizers
Several frequently asked questions surround non-vacuum type N sterilizers. For instance, many users wonder what types of materials should not be sterilized in non-vacuum autoclaves. Generally, porous materials, fabrics, and heat-sensitive devices should be avoided to prevent damage or incomplete sterilization.
Another common inquiry relates to how non-vacuum sterilizers ensure efficacy. These devices utilize precise temperature and time parameters calibrated to achieve adequate sterilization through steam exposure, making routine validation of cycles essential for maintaining effectiveness.
Non-vacuum sterilizers may not penetrate certain materials effectively.
Ensure cycles are monitored for consistent efficacy.
Establish a schedule for routine upkeep and inspection.
Maintenance practices essential for longevity include regular cleaning, calibration of settings, and periodic professional servicing. Facilities that invest time in these practices will not only enhance the performance of their sterilizers but also prolong their operational lifespan, ultimately saving resources.
Future trends in sterilization technology
The landscape of non-vacuum sterilization technologies is rapidly evolving. Innovations in materials and designs aim to enhance the efficiency and effectiveness of sterilization processes. For instance, the introduction of smart technology and IoT integration in sterilizers is expected to revolutionize monitoring and control, thus optimizing cycles and energy consumption.
Furthermore, the role of advanced technology continues to expand in improving sterilization efficacy. Modern sterilizers are now designed to provide detailed logging and reporting capabilities, enabling better compliance with regulatory guidelines and operational transparency. Facilities are increasingly leveraging data analytics to adjust cycles based on real-time insights.
Incorporating IoT for enhanced monitoring and operational efficiency.
Using real-time insights to optimize sterilization cycles.
Investments in research for advanced materials and design.
Emerging regulations are also shaping the future of non-vacuum sterilization, as regulatory bodies emphasize stricter compliance. Facilities need to stay ahead of these changes to ensure their sterilization practices remain compliant, informing decisions on equipment procurement and operational practices.