Understanding the Human Subjects Research AMP Form
Overview of human subjects research
Human subjects research, a critical area of study, involves conducting investigations involving living individuals to gather data on health, behaviors, and social dynamics. This research is integral to advancing medical knowledge and enhancing public health strategies. In the U.S. alone, the National Institutes of Health (NIH) reported that over 60% of clinical studies involve human participants, underscoring the vast scope and necessity of this research type.
Key stakeholders in human subjects research encompass researchers, ethical review boards, funding agencies, and the participants themselves. Each plays a vital role in the research process, ensuring that studies are not only scientifically valid but also ethically sound. Importantly, ethical considerations are paramount as they address issues such as informed consent, participant safety, and the potential for coercion. Ethical guidelines, such as those mandated by the Belmont Report, ensure that respect for persons, beneficence, and justice are upheld in all human research endeavors.
Understanding the AMP form
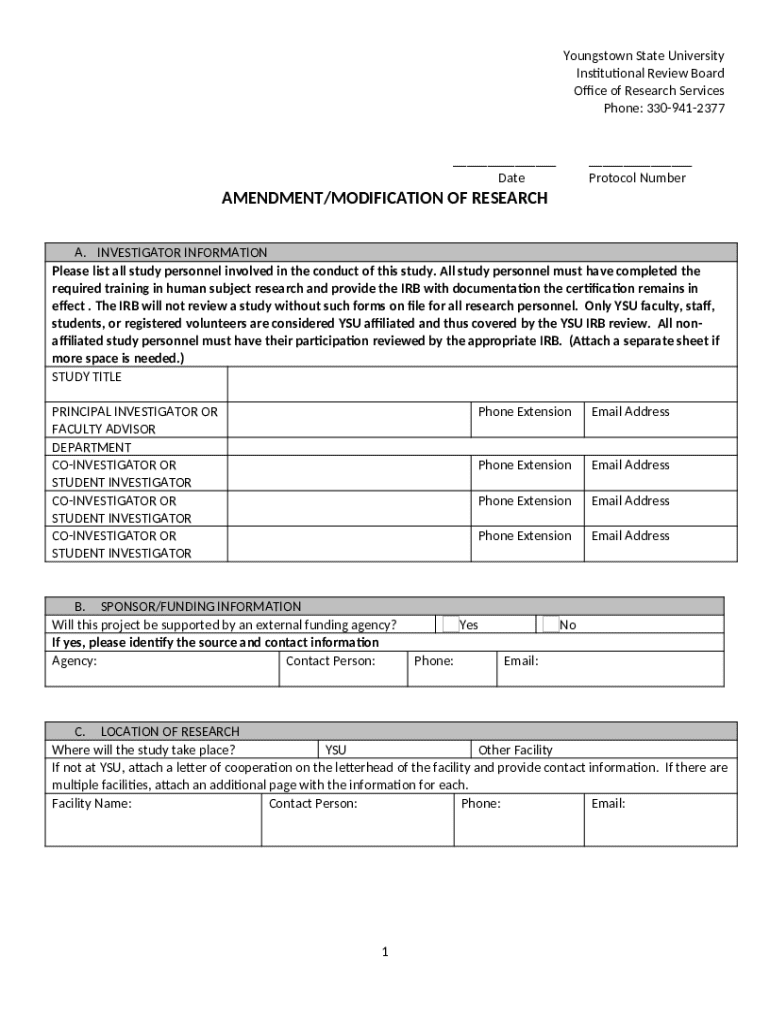
The Human Subjects Research AMP Form serves as a vital documentation instrument facilitating the ethical and regulatory submission of research proposals involving human participants. This form is designed to systematically capture essential details about a study, thereby ensuring compliance with federal and institutional regulations. It also serves as a communication tool between researchers and Institutional Review Boards (IRBs), promoting transparency and ethical accountability.
The significance of the AMP form cannot be overstated. It not only helps researchers outline their study's methodology and objectives but also ensures that participant rights and welfare are prioritized. The AMP form is required for most studies involving human subjects, particularly those that seek institutional funding or aim to publish findings in reputable journals. Consequently, familiarity with this form—and its requirements—becomes a crucial part of the research process.
Preparing to use the AMP form
Before submitting the AMP form, researchers must determine their eligibility. Typically, anyone conducting research involving human participants at an institution is required to submit this form. This includes university faculty, graduate students, and even affiliated researchers operating under institutional auspices.
However, some studies may fall under common exemptions. For instance, research involving existing data that does not personally identify individuals may not require the complete AMP form submission. Additionally, studies that solely involve educational practices or the use of authorized public dataset platforms might be exempt. Researchers should always consult with their institution's guidelines to clarify any uncertainties regarding submission requirements.
Detailed instructions for completing the AMP form
Completing the AMP form involves several critical sections that must be filled out carefully to ensure an accurate representation of the proposed research. First, the Participant Information section requires basic demographics and eligibility criteria for inclusion in the study. This provides a clear overview of who will participate, as well as key characteristics relevant to the research.
Next is the Study Description and Objectives section, where researchers must articulate their research question and specify the study's aims. Following this is the Informed Consent Procedures section, which details how researchers will ensure that participants understand their involvement, including potential risks, benefits, and their right to withdraw at any time. The Data Privacy and Participant Confidentiality section is equally important, detailing how researchers will protect participant data and ensure anonymity.
Provide clarity by defining specific terminologies related to your research.
Ensure that all sections are filled out comprehensively and accurately.
Use layman's terms when possible to enhance understanding for all audiences.
Review guidelines to avoid common mistakes such as missing signatures or incomplete data.
Submitting the AMP form
Submission methods for the AMP form vary by institution, but commonly accepted avenues include submitting via an online portal, email, or physical mailing. Institutions often provide specific online platforms tailored for efficient submissions. It’s crucial that researchers follow the institutional protocol to ensure their submission is received and reviewed promptly.
Timeline for review and approval can vary, generally taking anywhere from a few weeks to several months depending on the study's complexity and the number of submissions being processed. If researchers do not receive feedback in the expected timeframe, they should reach out to the IRB office or research administration for clarification.
Modifications and updates to the AMP form
If changes to the research study occur after submitting the AMP form, researchers must know when and how to request modifications. Common alterations might include changes in study design, participant recruitment strategies, or data management plans. Researchers can submit a Modification Request form to their IRB, detailing the adjustments and justifications for the changes.
Continuing reviews are required for ongoing studies to ensure they still align with ethical standards. Researchers must also report any adverse events that occur during the study promptly. This vigilance not only protects participants but also affirms the researcher's commitment to ethical study conduct.
Protecting human subjects in research
The legal and regulatory framework governing human subjects research is multifaceted, with laws such as the Common Rule and the Belmont Report providing foundational ethical guidelines. The Institutional Review Board (IRB) plays a pivotal role in this framework, acting as an independent entity that reviews research proposals to ensure participant safety and ethical compliance before a study can commence.
Resources for finding an IRB can include institutional directories, or national databases that provide IRB information. Researchers often have common questions, such as 'Does my research require IRB approval?' and 'What happens if I’m working with multiple IRBs?' The answer to the first lies in understanding whether the research involves human subjects as defined by federal guidelines. For multiple IRBs, researchers must communicate well and ensure each board has the necessary information to comply with their respective protocols.
Tools and resources available through pdfFiller
pdfFiller offers several interactive tools that can enhance the experience of completing the AMP form. Using its PDF editing capabilities, users can create and edit forms directly within the platform. This feature allows researchers to collaborate with team members efficiently, ensuring that all necessary sections are addressed and that collective input enhances the form’s clarity and quality.
Additionally, eSigning the AMP Form through pdfFiller simplifies the process, making it quicker to obtain necessary permissions before submission. The platform also provides templates for related forms, ensuring researchers can streamline their documentation processes without having to start from scratch.
Language assistance and accessibility
When it comes to inclusivity in research, resources for non-English speakers are crucial. Institutions and platforms like pdfFiller often provide multilingual support, making the AMP form accessible for a diverse participant pool. These resources might include language translation services or multilingual form templates.
pdfFiller's accessibility features also ensure it caters to users with varying needs – from screen reader compatibility to adjustable UI elements that suit those with disabilities. This commitment to accessibility reflects a broader understanding of the importance of inclusivity in research practices.
Addressing common user questions
Users often seek guidance on how to efficiently access pdfFiller’s AMP Form resources. The platform typically offers quick-access menus and support articles that direct users to the necessary forms and tools quickly. Assistance options for using the AMP form include live chat support, tutorials, and FAQ sections that address frequent queries, while troubleshooting guides help resolve common issues.
Tracking required training and documentation, such as CITI training updates, can also be simplified through pdfFiller, allowing researchers to maintain compliance with ethical training requirements seamlessly. This level of organization supports efficient research operations, ensuring researchers can focus on their studies without getting bogged down by administrative tasks.
Final submission checklist
To ensure the submission of a complete and thorough AMP form, researchers should create a final submission checklist. Key documents often included in this submission are copies of informed consent documents, data management plans, and research instruments such as surveys or interview guides.
Frequent items that researchers may overlook include ensuring that all required signatures are present, confirming that all sections are fully completed, and verifying that participant recruitment criteria align with the described methodology. It is essential for researchers to review their form one final time before submission to improve their chances of a smooth approval process.