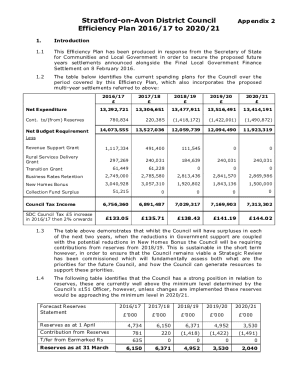
Treatment compassionate use template form: A comprehensive guide
Understanding compassionate use and its importance
Compassionate use refers to the provision of investigational medical products to patients with serious or lifethreatening conditions who lack alternative treatment options. This practice holds immense significance in healthcare as it can grant patients access to potentially lifesaving therapies before the completion of formal clinical trials. While clinical trials are designed to rigorously evaluate drug safety and efficacy, compassionate use provides a vital bridge for patients who may not survive long enough to wait for these trials to conclude.
The ethical perspective driving compassionate use centers on the principle of beneficence, which emphasizes the obligation to act in the best interest of patients. For those suffering from chronic or terminal illnesses, compassionate use programs can represent a beacon of hope, allowing access to innovative treatments that may significantly alter their prognosis or improve their quality of life.
Overview of the compassionate use template form
A compassionate use template form is a standardized document that simplifies the application process for accessing investigational treatments. Utilizing such a template ensures that all necessary information is captured systematically, facilitating efficient processing by healthcare providers and pharmaceutical companies. The uniformity of the template allows for faster evaluations and better communication between all parties involved.
Key components of this form usually include sections for personal information, treatment requested, medical history, and supporting documentation. Standardizing these elements helps to reduce errors and ambiguities that can lead to delays in obtaining critical treatments for patients.
How to fill out the compassionate use template form
Completing the compassionate use template form accurately is crucial for a successful submission. Here’s a step-by-step guide to assist in this process:
Collect necessary information: Gather all relevant documents, including medical records, physician statements, and any previous treatment history prior to filling out the form.
Filling in patient information: Enter accurate details such as name, date of birth, and contact information to ensure clear identification.
Detailing the treatment requested: Clearly specify the investigational product and dosage to avoid confusion during review.
Inclusion of supporting documents: Attach any required medical documentation that supports the need for compassionate use.
Reviewing the form for accuracy: Before submission, double-check all entries for completeness and correctness to reduce chances of delays.
Editing and customizing your template
pdfFiller offers a robust platform for editing compassionate use template forms. Its intuitive editing features allow users to personalize forms easily, ensuring they meet specific needs. Users can adjust text, add notes, and even include medical graphics if necessary.
Moreover, pdfFiller supports collaboration among team members. By inviting colleagues to review or edit the form, everyone involved can contribute, ensuring that all relevant expertise is leveraged in the patient’s best interest.
Signing and managing the document
Using pdfFiller, adding electronic signatures to the compassionate use template form is seamless. Users can sign documents using a stylus, mouse, or by entering their name, ensuring that the document is legally binding. Electronic signatures hold validity in most jurisdictions, removing barriers often posed by physical paper transactions.
Furthermore, pdfFiller streamlines document management. Users can organize their forms efficiently, facilitating quick access and tracking submission statuses. This enhances the overall process for both health practitioners and patients, allowing for proactive follow-ups on applications.
Specific instructions for different scenarios
Submissions to regulatory agencies can differ from those for healthcare providers. When submitting a compassionate use request to regulatory bodies, it’s advisable to adhere closely to specific guidelines and documentation requirements set forth by each agency. These often detail the necessary clinical information and risk assessment before approval can be granted.
For healthcare providers filling out forms on behalf of patients, familiarity with institutional protocols is key. Providers must ensure all relevant information is captured while observing privacy regulations and obtaining necessary consents from patients.
FAQs and troubleshooting common issues
When navigating the compassionate use process, questions may arise regarding eligibility criteria, timelines, and required documentation. It's essential to clarify these points ahead of time to avoid delays. Additionally, regulatory bodies may be reached out to for clarifications concerning updates and current practices.
For technical challenges while using pdfFiller, common issues such as access problems or form submission errors can often be resolved with simple troubleshooting steps. For persistent issues, users can consult pdfFiller’s customer support for assistance.
Best practices for a successful submission
To ensure a smoother submission process, consider these dos and don’ts:
Use the compassionate use template form for all requests to standardize information provided.
Engage with pharmaceutical companies proactively to discuss potential treatments and their availability.
Submit incomplete forms; ensure all fields are filled and required attachments are included.
Neglect to follow up with regulatory bodies or drug manufacturers to check the status of your request.
Building effective relationships with pharmaceutical companies can aid in accessing compassionate use drugs. Open dialogue and clear expressions of urgency can often expedite the process, making it beneficial for both patients and healthcare providers.
Popular resources related to compassionate use
Several resources are available to assist in understanding and navigating the compassionate use process. Related forms and templates, such as pre-approval worksheets and patient consent forms, can provide additional guidance in preparing for submissions.
Additionally, there are numerous articles and case studies available online that highlight successful compassionate use stories, providing insights into effective strategies or common pitfalls encountered by others.
Contact information and support
For users who experience difficulties while using pdfFiller, the platform offers various customer support options, including live chat and comprehensive FAQs. This ensures that any issues encountered can be resolved promptly, enabling users to continue their compassionate use efforts seamlessly.
Expert insights are also accessible for those in need of personalized assistance related to compassionate use. Professionals and consultants familiar with the regulatory landscape can often provide valuable guidance tailored to each unique situation.