Comprehensive Guide to the MTN-030 LDMS Tracking Sheet Form
Understanding the MTN-030 LDMS tracking sheet form
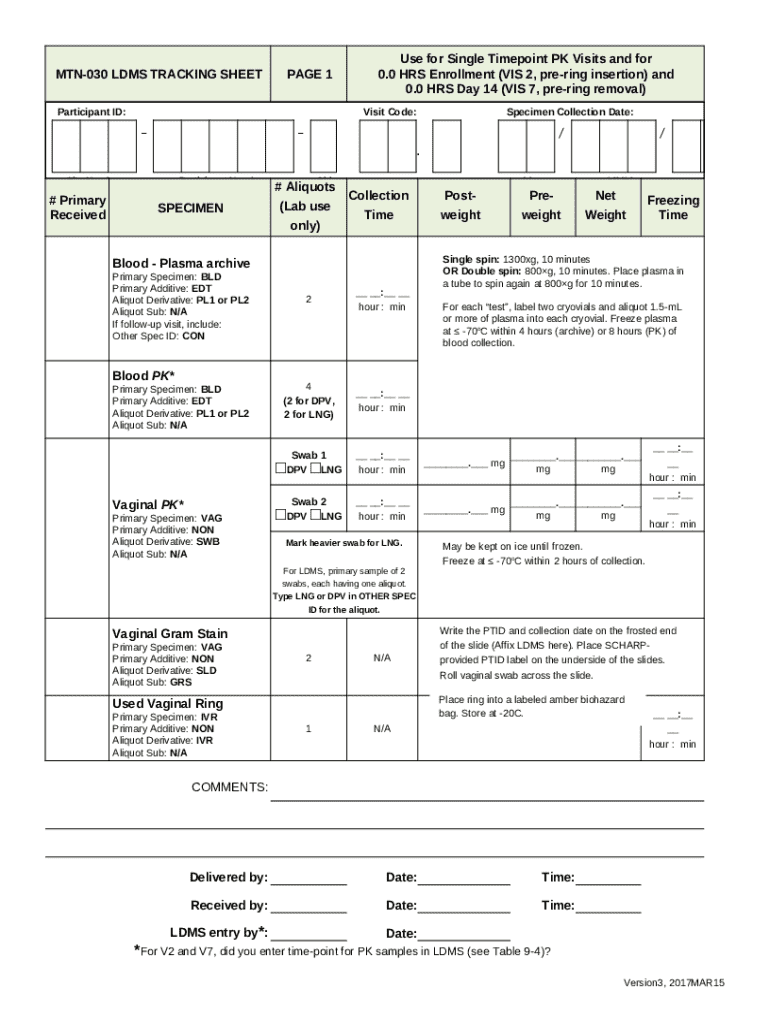
The MTN-030 LDMS Tracking Sheet is an essential tool in the context of clinical trials, specifically designed to track crucial data related to specimen and participant information. This form serves as a comprehensive record that ensures all necessary data from participant recruitment to sample analysis is meticulously captured and organized. Utilizing this tracking sheet not only enhances data integrity but also supports compliance with regulatory requirements.
Tracking sheets are pivotal in clinical trials since they bridge the gap between raw data collection and actionable insights. They are instrumental in facilitating monitoring, reporting, and data analysis processes within the clinical research ecosystem. LDMS, or Laboratory Data Management Systems, play a foundational role by allowing researchers to input, manage, and retrieve data efficiently.
Key features of the MTN-030 LDMS tracking sheet
The MTN-030 LDMS Tracking Sheet boasts several interactive and collaborative features designed for enhanced usability. One of the standout aspects of this tracking sheet is its interactive tools for data entry, enabling researchers to input information effortlessly while minimizing errors. This feature is vital for maintaining accurate and up-to-date records, which are crucial in the fast-paced environment of clinical trials.
Collaboration is another key feature that sets this tracking sheet apart. It allows team members to work together in real time, facilitating seamless communication and information sharing. Furthermore, the cloud-based accessibility ensures that users can access and update the document from anywhere, making it particularly convenient for distributed teams.
How to fill out the MTN-030 LDMS tracking sheet
Filling out the MTN-030 LDMS Tracking Sheet is a straightforward process when adhering to a systematic approach. Begin by carefully inputting patient information, which includes demographic details and unique identifiers necessary for tracking. Following this, document the sample collection information, specifying the types of samples collected, methods used, and processing protocols employed.
Additionally, keeping track of key dates is crucial; this includes enrollment dates, collection dates, and any relevant tracking milestones. Each of these components contributes to a comprehensive overview of the project, enabling researchers to monitor progress effectively. For accuracy, employing double-checking techniques and ensuring data consistency is essential—especially in relation to patient identifiers and protocol compliance.
Customizing the MTN-030 LDMS tracking sheet
The MTN-030 LDMS Tracking Sheet is not a one-size-fits-all solution; it offers varying options for customization to better fit the specific needs of your research team. Polling input from team members on essential data points can guide necessary modifications, promoting a tailored approach. Users can easily edit and modify the template to add sections or fields that capture localized metrics or specific requirements relevant to their study.
Utilizing pdfFiller’s editing tools enhances personalization, offering a user-friendly interface to adjust the form according to your project needs. This flexibility is particularly beneficial for teams looking to manage diverse trials or integrate special reporting commitments.
Signing and collaborating on the MTN-030 LDMS tracking sheet
Signing the MTN-030 LDMS Tracking Sheet need not be a cumbersome task; pdfFiller provides secure eSigning options that are compliant with regulatory standards. Users can effectively streamline the signature process, allowing for rapid approvals without sacrificing security. This is especially valuable in situations that require swift action on document management.
Collaboration is further advanced within the tracking sheet, with features that facilitate real-time input from multiple team members. This fosters an environment of enhanced communication and allows for document version management, which is essential to track changes and ensure everyone is working with the most current version of the research data.
Managing your MTN-030 LDMS tracking sheet
Once the MTN-030 LDMS Tracking Sheet is completed, efficient management is key to maintaining the integrity of your data. Saving and storing the completed form in a secure cloud environment ensures that it is both retrievable and protected from loss. Additionally, sharing the document with stakeholders, such as researchers or regulatory bodies, can be done securely, ensuring compliance and confidentiality.
Keeping track of changes over time is vital for transparency in clinical trials. pdfFiller’s version control and history features log edits and updates, which can be particularly beneficial during audits or reviews. These tools guarantee that your research documentation remains rigorous and in line with the best practices of clinical trial management.
Troubleshooting common issues with the MTN-030 LDMS tracking sheet
Like any document, users may encounter common issues while using the MTN-030 LDMS Tracking Sheet. These may include difficulties in data input, navigating the editing features, or understanding the documentary requirements. Addressing these hurdles promptly is crucial to maintaining project timelines.
FAQs associated with the form’s usage are valuable resources that can provide immediate help to users. Should further complications arise, pdfFiller offers robust customer support to assist in resolving any persistent matters. This support network reflects a commitment to ensuring users can harness the full potential of the MTN-030 LDMS Tracking Sheet.
Best practices for using the MTN-030 LDMS tracking sheet in clinical trials
Implementing the MTN-030 LDMS Tracking Sheet effectively requires adherence to best practices that ensure compliance with regulatory standards. Research teams should emphasize regular training on the features of the sheet, so all members are familiar with its functionalities and the importance of accurate data entry.
Additionally, fostering a culture of accountability can promote diligent data management and tracking. By ensuring everyone understands their roles and responsibilities concerning data entry, the overall efficiency of the trial is enhanced. Consistent reviews of the captured data also support quality control, which is indispensable for obtaining credible results in clinical trials.
Case studies: Success stories using the MTN-030 LDMS tracking sheet
Numerous clinical trials have demonstrated the effectiveness of the MTN-030 LDMS Tracking Sheet in driving successful project outcomes. For instance, a recent trial focused on vaccine development utilized this tracking sheet to standardize data collection, significantly reducing errors and enhancing participant follow-up rates. Feedback from research teams highlighted the ease of access and real-time updates as critical contributors to their success.
Lessons learned from these real-world applications indicate that the MTN-030 LDMS Tracking Sheet is not merely a tool but rather an integral part of the research framework that promotes efficiency, collaboration, and data integrity. By analyzing these success stories, teams can glean insights into best practices and strategies that enhance trial management.
Future developments and enhancements for the MTN-030 LDMS tracking sheet
The MTN-030 LDMS Tracking Sheet is poised for evolution, with several upcoming features being developed for enhanced user experience. Continuous user input and feedback are integral to shaping future updates, ensuring that developers are responsive to the needs of the scientific community. Anticipated improvements may include advanced analytical tools for data interpretation and enhanced collaboration features aimed at streamlining communication among research teams.
This commitment to improving the MTN-030 LDMS Tracking Sheet reflects pdfFiller’s dedication to delivering solutions that are not only comprehensive but also adaptable to the evolving landscape of clinical research.