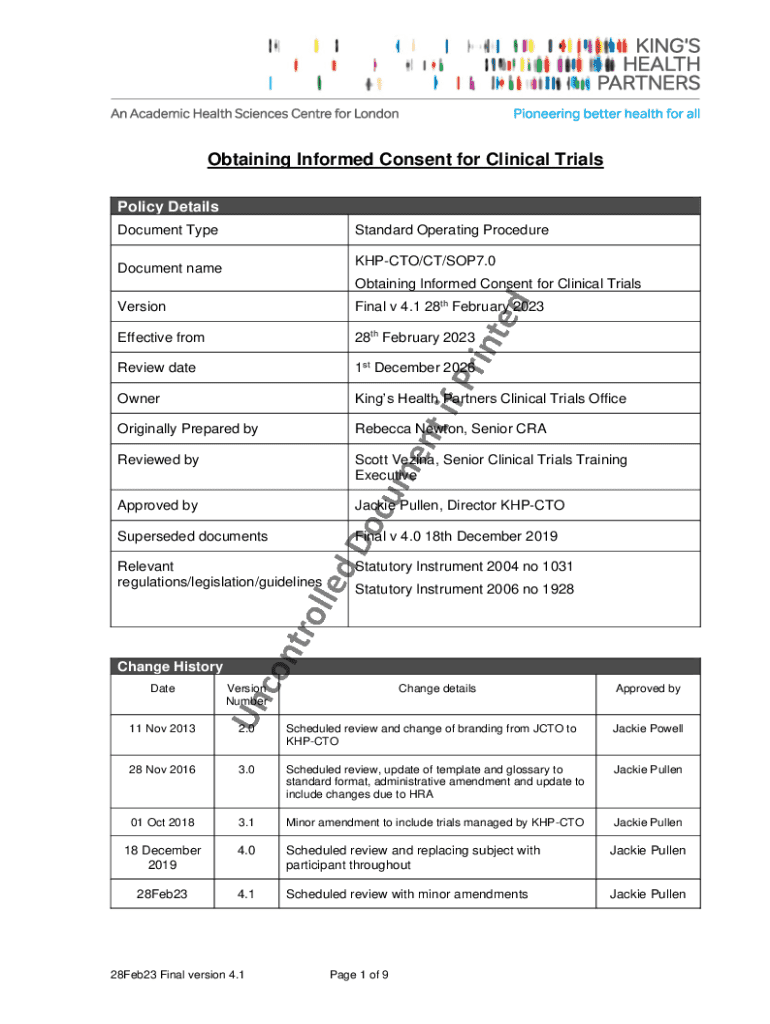
Get the free SOP: 7. Obtaining Informed Consent for Clinical Trials
Get, Create, Make and Sign sop 7 obtaining informed



How to edit sop 7 obtaining informed online
Uncompromising security for your PDF editing and eSignature needs
How to fill out sop 7 obtaining informed

How to fill out sop 7 obtaining informed
Who needs sop 7 obtaining informed?
SOP 7: Obtaining Informed Consent Form
Understanding SOP 7: An overview
SOP 7 refers to the Standard Operating Procedure guiding the process of obtaining informed consent. This procedure is crucial across various fields, most notably in medical practices and research settings, where individuals must be fully aware of what they are consenting to before proceeding. Without a clear understanding, participants may unknowingly place themselves at risk or compromise their rights.
Key components of the informed consent form
An informed consent form must encompass several critical components to effectively communicate essential information to participants. Each element plays a pivotal role in ensuring participants understand the purpose, risks, and implications of their involvement.
Inclusion of these components can be guided by templates, specific to the field of application. These templates often encapsulate the core requirements while providing flexibility for customization.
Steps to create an effective informed consent form
Creating a robust informed consent form is critical for ensuring clarity and compliance. A methodical approach is vital, and the process can be streamlined into several key steps.
Tips for ensuring clarity and comprehensibility
To maximize understanding, the informed consent form should be free of complex language and legalese. Simple, clear communication is essential in facilitating participant comprehension.
Interactive tools and features on pdfFiller
pdfFiller offers a powerful suite of tools specifically designed to create and edit informed consent forms effectively. Users benefit from an intuitive interface that simplifies document customization.
Best practices for signing and managing the informed consent form
Once the informed consent form is created, signing and managing the document requires adherence to best practices to enhance security and compliance.
Handling challenges with informed consent forms
Challenges may arise during the informed consent process, including misunderstandings about the content and instances of refusal to sign.
Case studies: Successful implementation of informed consent procedures
Real-life examples highlight the importance of informed consent in practice. Organizations that have successfully implemented structured consent procedures report higher rates of participant satisfaction and trust.
Regulatory compliance and ethical considerations
Adhering to regulatory frameworks is essential when developing informed consent forms. Compliance with regulations such as HIPAA and GDPR ensures the protection of participants' rights and privacy.
Future trends in informed consent practices
Emerging technologies will influence the informed consent landscape significantly. Innovations such as blockchain for secure data management and artificial intelligence for personalized consent processes are gaining traction.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send sop 7 obtaining informed for eSignature?
How do I fill out sop 7 obtaining informed using my mobile device?
How do I edit sop 7 obtaining informed on an iOS device?
What is sop 7 obtaining informed?
Who is required to file sop 7 obtaining informed?
How to fill out sop 7 obtaining informed?
What is the purpose of sop 7 obtaining informed?
What information must be reported on sop 7 obtaining informed?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

























