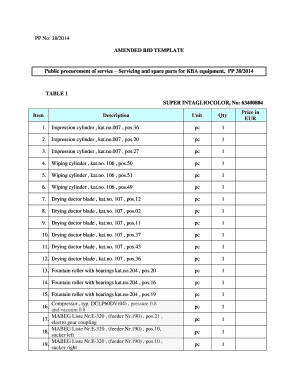
Get the free D(-)-Sorbitol GPR RECTAPUR GPR RECTAPUR
Get, Create, Make and Sign d--sorbitol gpr rectapur gpr



Editing d--sorbitol gpr rectapur gpr online
Uncompromising security for your PDF editing and eSignature needs
How to fill out d--sorbitol gpr rectapur gpr

How to fill out d--sorbitol gpr rectapur gpr
Who needs d--sorbitol gpr rectapur gpr?
A detailed guide to -sorbitol GPR Rectapur GPR form
Understanding -sorbitol
D-sorbitol, a sugar alcohol that derives from glucose, has a clear chemical structure denoted by the formula C6H14O6. This compound appears as a white crystalline substance, often utilized for its sweetening capabilities and hygroscopic properties. As a polyol, it plays a crucial role across various domains, providing both functional and health benefits.
In addition to its applications, d-sorbitol boasts health benefits, like low glycemic index ratings, making it suitable for diabetics. Nevertheless, excessive consumption can lead to digestive issues, such as bloating or laxative effects, which users should consider.
Overview of GPR (General Product Requirement)
General Product Requirements (GPR) define the quality and safety standards that products must meet before reaching consumers. Compliance with GPR is vital in product development, ensuring that substances like d-sorbitol align with health and safety regulations.
The specifications set forth in GPR are crucial for manufacturers and distributors, emphasizing the necessity for rigorous testing and documentation, especially when designing products that incorporate d-sorbitol.
Specifics of the GPR Rectapur
GPR Rectapur signifies a set of prescribed standards for various substances, including d-sorbitol. The relationship between GPR Rectapur and d-sorbitol lies in its detailed guidelines, which dictate the acceptable forms and purity levels for this ingredient, which is critical for maintaining high safety and quality benchmarks.
Utilizing Rectapur standards means manufacturers can hold a competitive edge by showcasing their commitment to product safety, thus enhancing consumer trust and satisfaction.
Filling out the GPR form for -sorbitol
Completing the GPR form for d-sorbitol involves several detailed steps to ensure that all required information is accurately represented. Here’s a step-by-step approach to streamline the process.
Following these steps carefully can simplify the process and enhance the likelihood of a successful submission.
Managing and reviewing your GPR form submission
Once the d-sorbitol GPR form is filled out, thorough review becomes essential. This process involves several best practices to ensure accuracy and compliance.
Utilizing collaboration features available in pdfFiller allows you to invite team members to review the document, facilitating real-time tracking of changes and comments for improved accuracy.
Troubleshooting common issues
While filling out the d-sorbitol GPR form, you may encounter a variety of challenges. Understanding common errors can expedite resolution.
Additionally, having a list of FAQs regarding GPR submissions can offer guidance and help streamline your process. Ensure you consult these resources regularly for the latest updates.
Understanding the regulatory framework
Regulatory bodies like the FDA and EMA govern the use of d-sorbitol, establishing clear guidelines that companies must follow for compliance. Keeping track of these regulations is vital for maintaining viability in various markets.
By staying informed and ensuring documentation aligns with current regulations, companies can mitigate risks and maintain product quality.
Advanced tips for managing your -sorbitol documents
Effective document management for d-sorbitol incorporates advanced strategies for enhanced accessibility and control. Utilizing cloud-based platforms like pdfFiller offers numerous advantages.
Implementing these strategies ensures that your documentation remains organized, secure, and accessible for future reference.
Real-life case studies
Analyzing success stories can provide valuable insights into efficient practices for d-sorbitol documentation. Teams utilizing pdfFiller have reported significant improvements in their submission processes.
These real-life examples serve as a reminder of the importance of thorough documentation and the role that tools like pdfFiller play in achieving it.
Conclusion
Diligence in GPR documentation is not merely a bureaucratic requirement; it is pivotal in ensuring product integrity and consumer trust. The d-sorbitol GPR Rectapur form requires accurate data entry, compliance with safety standards, and commitment to quality.
The comprehensive features offered by pdfFiller empower teams to streamline this complex process, leading to enhanced efficiency, collaboration, and ultimately, better product outcomes. Leveraging pdfFiller's tools equips individuals and organizations to manage their d-sorbitol documents professionally, meeting industry standards while ensuring accuracy and compliance.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in d--sorbitol gpr rectapur gpr?
How do I make edits in d--sorbitol gpr rectapur gpr without leaving Chrome?
How do I fill out d--sorbitol gpr rectapur gpr on an Android device?
What is d--sorbitol gpr rectapur gpr?
Who is required to file d--sorbitol gpr rectapur gpr?
How to fill out d--sorbitol gpr rectapur gpr?
What is the purpose of d--sorbitol gpr rectapur gpr?
What information must be reported on d--sorbitol gpr rectapur gpr?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.