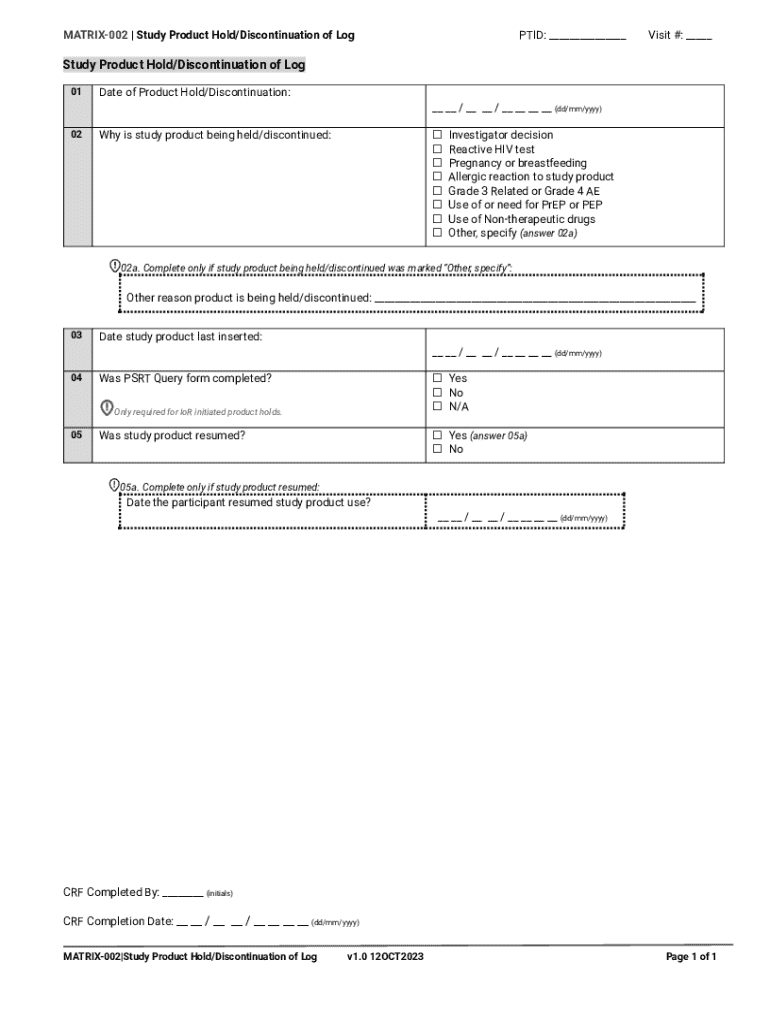
Get the free MATRIX-002 Study Product Hold/Discontinuation of Log
Get, Create, Make and Sign matrix-002 study product holddiscontinuation



Editing matrix-002 study product holddiscontinuation online
Uncompromising security for your PDF editing and eSignature needs
How to fill out matrix-002 study product holddiscontinuation

How to fill out matrix-002 study product holddiscontinuation
Who needs matrix-002 study product holddiscontinuation?
Matrix-002 Study Product Holddiscontinuation Form: A Comprehensive Guide
Understanding the Matrix-002 Study Product
The Matrix-002 study is a pivotal clinical research initiative aimed at evaluating the efficacy and safety of novel therapeutic approaches. This study encompasses various phases, focusing on rigorous analysis of participant outcomes and reactions to the product under investigation. Ensuring meticulous documentation and adhering to regulatory standards are crucial for the success of such studies.
Key objectives of the Matrix-002 study include understanding dosage effects, patient adherence, and long-term health impacts related to the treatment. By achieving these objectives, researchers can substantiate claims regarding the product's clinical advantages, contributing significantly to medical literature.
In this context, the Holddiscontinuation Form plays a vital role in ensuring all actions taken during the study are well-documented. This form is critical for maintaining compliance with ethical standards and regulatory requirements, ultimately protecting both the participant’s welfare and the integrity of the research.
What is the Holddiscontinuation Form?
The Holddiscontinuation Form is an essential document that researchers must complete when a participant in the Matrix-002 study needs to pause or discontinue their involvement. The primary purpose of this form is to provide a structured way to communicate the reasons for the hold or discontinuation, ensuring all relevant stakeholders can stay informed.
This form must be used in various situations, such as when a participant exhibits adverse reactions, wishes to withdraw, or when a study protocol necessitates temporary cessation. Utilizing the Holddiscontinuation Form enhances transparency and accountability, crucial aspects for maintaining the quality and rigor of clinical research.
Furthermore, the Holddiscontinuation Form aids in protecting participant rights and well-being, making it a cornerstone of compliance with ethical research standards.
Who Needs to Use the Holddiscontinuation Form?
The responsibility of submitting the Holddiscontinuation Form primarily falls on clinical trial coordinators and investigators overseeing the Matrix-002 study. These individuals must ensure that any discontinuations are promptly reported to maintain the study's integrity.
In addition, research teams that monitor participant recruitment, safety, and data integrity play a pivotal role in facilitating this process. Other stakeholders, like ethics committees and regulatory bodies, are also affected by the changes communicated through the Holddiscontinuation Form, which aids in their decision-making processes.
Step-by-Step Guide to Completing the Holddiscontinuation Form
Successfully completing the Holddiscontinuation Form requires careful attention to detail. Here’s a streamlined guide to help you through the process.
Editing and Managing the Holddiscontinuation Form
Editing the Holddiscontinuation Form can be accomplished easily via pdfFiller. This cloud-based platform allows users to access the form anytime, ensuring that even last-minute edits can be made without hassle.
Using pdfFiller, you can navigate to the form, make necessary adjustments, and add comments or explanations as needed. Furthermore, it is imperative to save changes securely to avoid any loss of data. The platform offers various tools to assist in documentation security, ensuring your changes remain secure and easily tracked.
eSigning the Holddiscontinuation Form
eSigning is an integral part of the Holddiscontinuation Form submission process, as it provides an added layer of authenticity and security. Ensuring that the necessary parties (typically the investigator and the participant) sign the form electronically confirms that all information is acknowledged and accepted.
To leverage the eSigning feature on pdfFiller, users should follow these steps: First, open the form, then navigate to the signature section, and use the platform's eSignature tool to add your digital signature. This feature is designed to be user-friendly and ensures that your submissions conform to legal requirements.
Collaboration tools available for teams
Teams involved in the Matrix-002 study can greatly benefit from the collaborative tools offered by pdfFiller. These tools facilitate smooth collaboration and allow for contributions from multiple stakeholders.
To share the Holddiscontinuation Form with team members, users can utilize pdfFiller's sharing capabilities, which allow team members to collaborate in real-time. Additionally, the platform's interactive features enable team members to comment, suggest edits, and track changes, ensuring everyone remains on the same page throughout the process.
FAQs on the Holddiscontinuation Form
Given the importance of the Holddiscontinuation Form, it is common for users to have questions regarding its usage. Below are frequently asked questions to help clarify some common uncertainties.
Case studies and examples
Real-life scenarios can illuminate the importance of the Holddiscontinuation Form. For instance, one clinical trial faced challenges after a participant experienced unexpected side effects, leading to a swift submission of the Holddiscontinuation Form. Prompt action prevented further complications and ensured adherence to safety protocols.
This case demonstrates that using the Holddiscontinuation Form can facilitate effective communication between researchers and regulatory bodies, ultimately enhancing participant safety and maintaining study integrity.
Best practices in managing Holddiscontinuation Forms
Implementing best practices in managing the Holddiscontinuation Form is ideal for maintaining compliance and ensuring efficient documentation. Here are some tips:
Troubleshooting common issues
Common challenges when completing the Holddiscontinuation Form can include technical issues, misconception of entry requirements, and miscommunication among team members. Addressing these challenges proactively can prevent delays and stress.
Should you encounter a technical issue with pdfFiller, the platform offers dedicated support to troubleshoot. Additionally, maintain clear lines of communication with the team to ensure that everyone understands their roles and responsibilities when dealing with the form.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send matrix-002 study product holddiscontinuation for eSignature?
How do I fill out matrix-002 study product holddiscontinuation using my mobile device?
How do I edit matrix-002 study product holddiscontinuation on an iOS device?
What is matrix-002 study product holddiscontinuation?
Who is required to file matrix-002 study product holddiscontinuation?
How to fill out matrix-002 study product holddiscontinuation?
What is the purpose of matrix-002 study product holddiscontinuation?
What information must be reported on matrix-002 study product holddiscontinuation?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















