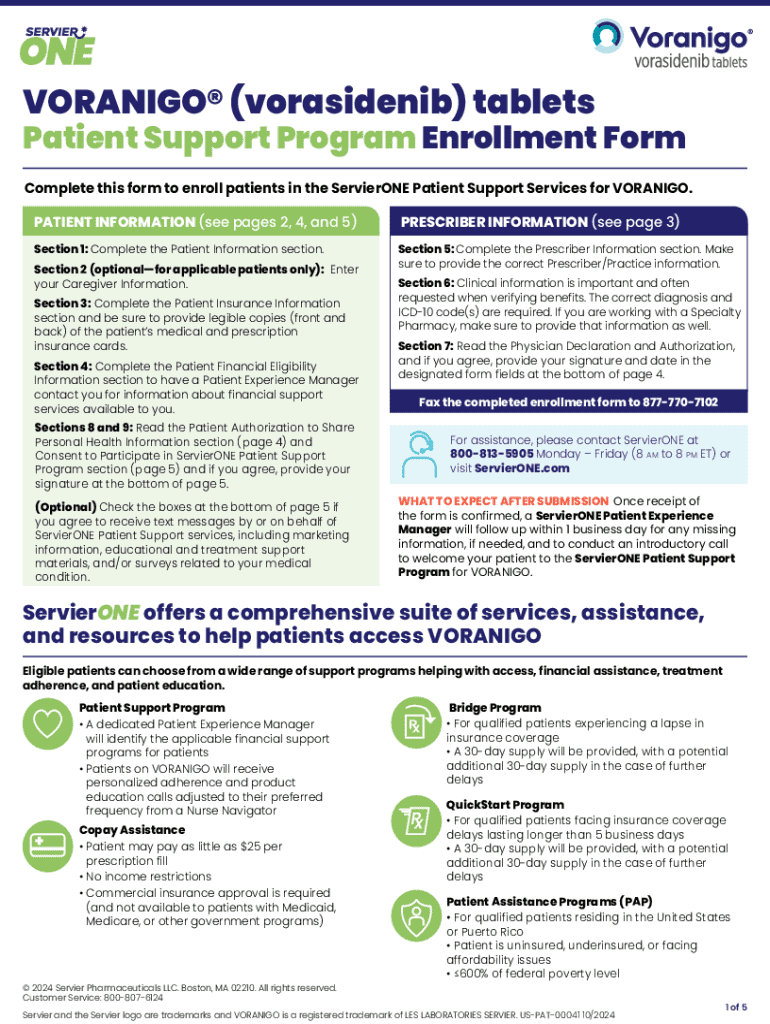
Get the free Vorasidenib (Voranigo) for Low-Grade Glioma (online only)
Get, Create, Make and Sign vorasidenib voranigo for low-grade



Editing vorasidenib voranigo for low-grade online
Uncompromising security for your PDF editing and eSignature needs
How to fill out vorasidenib voranigo for low-grade

How to fill out vorasidenib voranigo for low-grade
Who needs vorasidenib voranigo for low-grade?
Vorasidenib Voranigo for Low-Grade Form: A Comprehensive Guide
Understanding low-grade gliomas
Low-grade gliomas are a group of brain tumors that are classified as slow-growing and less aggressive than their high-grade counterparts. Often originating from glial cells, these tumors can be challenging to diagnose and treat due to their variable behavior and location within the brain. A significant factor in diagnosing and understanding these tumors is the presence of isocitrate dehydrogenase (IDH) mutations, which can assist in determining prognosis and potential treatment paths.
Common subtypes of low-grade gliomas include astrocytomas, oligodendrogliomas, and mixed gliomas, each presenting unique characteristics. Astrocytomas arise from astrocytes, which support and protect neurons. Oligodendrogliomas, on the other hand, originate from oligodendrocytes, which insulate neuronal axons. The low-grade forms of these tumors usually result in symptoms that develop gradually, often leading to a delay in diagnosis and treatment.
Vorasidenib overview
Vorasidenib is an innovative oral medication designed specifically to target and inhibit mutant IDH1 proteins found in many low-grade gliomas. This targeted approach helps in addressing the metabolic alterations created by these mutations, thus altering the tumor's environment while sparing healthy cells to a greater extent than traditional chemotherapy treatments. Vorasidenib’s precision in targeting specific pathways significantly reduces the risk of harmful side effects commonly associated with chemotherapy.
Complementing Vorasidenib is Voranigo, a supportive therapy that can enhance patient outcomes by maximizing the efficacy of Vorasidenib. While Vorasidenib primarily targets the genetic abnormalities within tumors, Voranigo focuses on symptom management and improving patient quality of life. Utilizing Voranigo in treatment protocols allows healthcare providers to offer a more holistic approach to care.
Approved uses and indications
Vorasidenib has received approval for the treatment of low-grade gliomas, specifically those characterized by IDH1 mutations. This approval is supported by robust clinical trials demonstrating Vorasidenib’s effectiveness in shrinking tumor size and prolonging progression-free survival. The FDA approved Vorasidenib based on trials that showed a statistically significant improvement in clinical outcomes for patients with this specific tumor type.
Eligibility for Vorasidenib treatment includes patients diagnosed with low-grade gliomas who express IDH1 mutations. Factors influencing eligibility can include the patient's overall health, the specific type of glioma, and previous treatments received. Healthcare teams use these considerations to tailor treatment recommendations to individual patient needs.
Treatment protocol for Vorasidenib
Vorasidenib is administered orally, typically in the form of tablets. The recommended starting dose is 100 mg once daily, with adjustments made based on individual patient response and tolerability. It is crucial for patients to adhere to the prescribed schedule, taking the medication consistently each day to maintain stable blood levels and maximize effectiveness.
During treatment with Vorasidenib, regular monitoring is essential to assess efficacy and identify any potential side effects early. Patients should expect to undergo frequent clinical assessments, including blood tests such as Complete Blood Count (CBC) and Comprehensive Metabolic Panel (CMP), as well as regular imaging studies to evaluate tumor response to treatment.
Side effects and management
Like any medication, Vorasidenib can lead to side effects, with common issues including nausea, fatigue, and headaches. Gastrointestinal symptoms like diarrhea or constipation, as well as skin reactions, may also occur. These side effects can impact a patient's quality of life, therefore managing them effectively is critical to maintaining adherence to treatment.
Healthcare professionals recommend various strategies for managing side effects. These may include dietary adjustments to mitigate gastrointestinal symptoms or specific medications to alleviate nausea. Patients should be encouraged to communicate openly with their healthcare team regarding side effects, ensuring timely and appropriate interventions when issues arise.
Understanding treatment goals
The primary objective of therapy with Vorasidenib is to control tumor growth and prolong patient survival while minimizing adverse side effects. Patients can expect to see a reduction in tumor size and improved overall health. Long-term management of low-grade gliomas often requires a comprehensive strategy, including close monitoring and adjustments to the treatment plan as necessary.
Furthermore, establishing clear treatment goals at the outset can enhance motivation and improve adherence. Patients and care teams should collaboratively define what success looks like, checking in regularly to evaluate progress and adjust expectations based on treatment outcomes.
Interactive tools and resources
Managing the complexities of treatment requires effective tools. pdfFiller offers a range of document management solutions tailored for patients and healthcare teams. Through pdfFiller, users can easily create and manage treatment consent forms, ensuring that all legal and medical documentation is in order before commencing therapy.
Additionally, pdfFiller allows for the secure editing and signing of medical documents remotely. Patients can easily access their medical records, request necessary updates, and collaborate with their healthcare teams through document sharing, ensuring everyone remains informed and engaged throughout the treatment process.
FAQs about Vorasidenib Voranigo
Patients often wonder if Vorasidenib can be combined with other treatments. While Vorasidenib provides targeted therapy, doctors may recommend supplemental treatments based on individual patient needs, including surgery or radiation. It’s important to discuss combination therapy options with your healthcare team for tailored guidance.
Lifestyle adjustments during treatment can also play a crucial role in recovery. Maintaining a balanced diet, engaging in regular, moderate exercise, and avoiding smoking may support overall health and enhance treatment efficacy. Additionally, understanding the impact of treatment on reproductive health is essential, leading many to inquire about the safety of Vorasidenib for pregnant or breastfeeding individuals. Current research suggests caution, and any decisions should involve comprehensive discussions with healthcare professionals.
Building emotional wellness during treatment
Receiving a diagnosis of low-grade glioma can have profound emotional impacts on both patients and their families. The importance of mental health cannot be understated, and many seek to build robust support networks to navigate the challenges of treatment. Engaging close friends or family members, or exploring support groups can provide emotional reassurance and practical assistance throughout the treatment journey.
Resources for patients and caregivers, including counseling services, online forums, and local support groups, can significantly aid emotional wellness. Being proactive in seeking support allows individuals to share their experiences and challenges, fostering connections that can lead to improved mental health and resilience during challenging times.
Ongoing research and future directions
The field of glioma research is rapidly evolving, with promising therapeutics and clinical trials underway involving Vorasidenib. Ongoing studies are exploring potential combinations with other agents and investigating biomarkers, which could help personalize treatment strategies. Advancements in genomic sequencing and precision medicine are paving the way for tailored therapeutic approaches that may enhance outcomes further.
Future directions for treating low-grade gliomas are bright, with various innovations on the horizon aimed at improving survival rates and quality of life. As research into Vorasidenib and similar agents continues, patients are encouraged to stay updated on new developments, as they might offer additional treatment opportunities.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send vorasidenib voranigo for low-grade to be eSigned by others?
How do I edit vorasidenib voranigo for low-grade on an iOS device?
How do I complete vorasidenib voranigo for low-grade on an Android device?
What is vorasidenib voranigo for low-grade?
Who is required to file vorasidenib voranigo for low-grade?
How to fill out vorasidenib voranigo for low-grade?
What is the purpose of vorasidenib voranigo for low-grade?
What information must be reported on vorasidenib voranigo for low-grade?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















