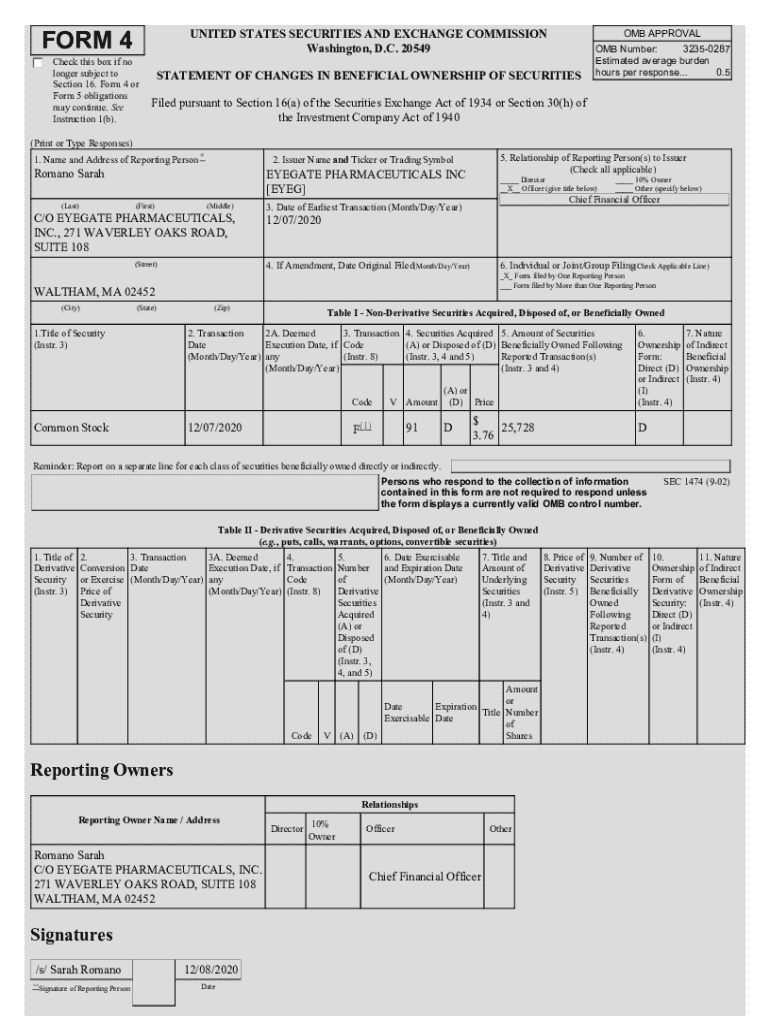
Get the free C/O EYEGATE PHARMACEUTICALS, INC
Get, Create, Make and Sign co eyegate pharmaceuticals inc



How to edit co eyegate pharmaceuticals inc online
Uncompromising security for your PDF editing and eSignature needs
How to fill out co eyegate pharmaceuticals inc

How to fill out co eyegate pharmaceuticals inc
Who needs co eyegate pharmaceuticals inc?
A Comprehensive Guide to the Co Eyegate Pharmaceuticals Inc. Form
Overview of Eyegate Pharmaceuticals Inc.
Eyegate Pharmaceuticals Inc. is a pioneering biopharmaceutical company focused on developing innovative treatments for ocular diseases. Founded to address unmet medical needs within the ophthalmic sector, Eyegate emphasizes the importance of research, development, and partnerships. Understanding the significance of the company’s forms is crucial for stakeholders, as they facilitate essential processes such as regulatory compliance, clinical trials, and collaborations with other organizations.
Within the pharmaceutical industry, several types of forms are utilized, including clinical trial applications, regulatory submissions, and partnership agreements. Each form serves a specific purpose and plays a vital role in ensuring effective communication between the company and relevant stakeholders, including regulatory bodies, investors, and clinical partners.
Understanding the Co Eyegate Pharmaceuticals Inc. Form
The 'co eyegate pharmaceuticals inc form' is a critical document utilized primarily for clinical development and partnership purposes. It encompasses essential information regarding clinical trials, partnerships, and operational integrity, thereby streamlining collaboration and regulatory interactions. This form serves as a foundational element in maintaining transparency between Eyegate and its stakeholders.
Specific use cases for the form include submitting trial protocols for approval, documenting compliance measures, and disclosing information pertinent to partnerships with academic institutions and healthcare organizations. Key stakeholders in the submission process include clinical research coordinators, regulatory affairs specialists, and senior management, all of whom play essential roles in ensuring accuracy and compliance.
Step-by-step instructions for filling out the Co Eyegate Pharmaceuticals Inc. form
Filling out the co eyegate pharmaceuticals inc form requires careful attention to detail. Here’s a structured approach to ensure completeness:
Editing and reviewing the Co Eyegate Pharmaceuticals Inc. form
Reviewing your completed co eyegate pharmaceuticals inc form is a critical step before submission. A thorough review can help identify typos, inconsistencies, and missing information. pdfFiller provides intuitive editing tools that make adjusting your form straightforward, allowing you to highlight areas needing changes or clarification.
Presenting a pristine document is vital in the pharmaceutical industry, where professionalism and attention to detail can influence stakeholder perception. The final appearance of your form should reflect the high standards upheld by Eyegate Pharmaceuticals Inc., enhancing readability and demonstrating commitment to quality.
eSignature features for the Co Eyegate Pharmaceuticals Inc. form
The eSignature process enables a streamlined method for signing the co eyegate pharmaceuticals inc form. Utilizing pdfFiller, users can eSign the form conveniently and securely, facilitating remote collaboration among stakeholders, even when they are in different geographic locations.
Understanding the legal validity of electronic signatures within pharmaceutical submissions is essential. In many jurisdictions, electronic signatures hold the same legal weight as handwritten signatures, provided they meet specific requirements. This flexibility not only expedites processes but also maintains thorough compliance with relevant regulations.
Collaboration tools with pdfFiller
pdfFiller's platform offers robust collaboration tools that enable teams to work closely on the co eyegate pharmaceuticals inc form. Features allow team members to leave comments, suggest edits, and track changes in real-time, fostering a collaborative atmosphere that enhances the quality and accuracy of submissions.
Inviting stakeholders to review and comment on the form is simple with pdfFiller’s shared access features. Additionally, users can manage multiple contributors efficiently while maintaining version control, ensuring that the most current and accurate document is always in circulation.
Managing submissions and tracking responses
Submitting the co eyegate pharmaceuticals inc form electronically is straightforward with pdfFiller. The platform offers comprehensive instructions that guide users through the submission process, allowing them to complete the necessary steps with confidence.
Once submitted, tracking the status of your form is facilitated through pdfFiller’s dashboard, which provides updates in real time. In situations where corrections are needed after submission, users can address these quickly through the same platform, maintaining a fluid workflow.
FAQs about the Co Eyegate Pharmaceuticals Inc. form
Understanding common queries can aid users in navigating the complexities of the co eyegate pharmaceuticals inc form. Frequently asked questions might include inquiries about the specific information required or how to amend submitted documents.
Data privacy is another concern, especially in the pharmaceutical industry. pdfFiller adheres to industry-standard practices for data management and protection, ensuring that users can fill out forms securely. For additional assistance, resources are readily available, offering users in-depth guidance as needed.
Case studies and testimonials
Real-world examples of successful form submissions stand testament to the effectiveness of the co eyegate pharmaceuticals inc form. Several users have reported streamlined processes and increased accuracy when utilizing pdfFiller for their document management needs.
Testimonials highlight the seamless experience users have had with pdfFiller, emphasizing how the platform’s features simplified what was previously a complex and time-consuming task. These benefits translate to enhanced productivity and fewer bottlenecks during critical clinical development phases.
Conclusion of the documenting process
The process of filling out the co eyegate pharmaceuticals inc form underscores the importance of accuracy and collaboration in clinical development. By leveraging resources like pdfFiller, teams can navigate the complexities of pharmaceutical documentation efficiently, ensuring that all necessary information is captured and submitted correctly.
Final thoughts hinge on the understanding that meticulous documentation practices not only foster regulatory compliance but also enhance cooperation among teams. Embracing platforms designed for seamless document management ultimately leads to better outcomes in research and development efforts.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit co eyegate pharmaceuticals inc from Google Drive?
How do I edit co eyegate pharmaceuticals inc in Chrome?
How do I fill out co eyegate pharmaceuticals inc on an Android device?
What is co eyegate pharmaceuticals inc?
Who is required to file co eyegate pharmaceuticals inc?
How to fill out co eyegate pharmaceuticals inc?
What is the purpose of co eyegate pharmaceuticals inc?
What information must be reported on co eyegate pharmaceuticals inc?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

























