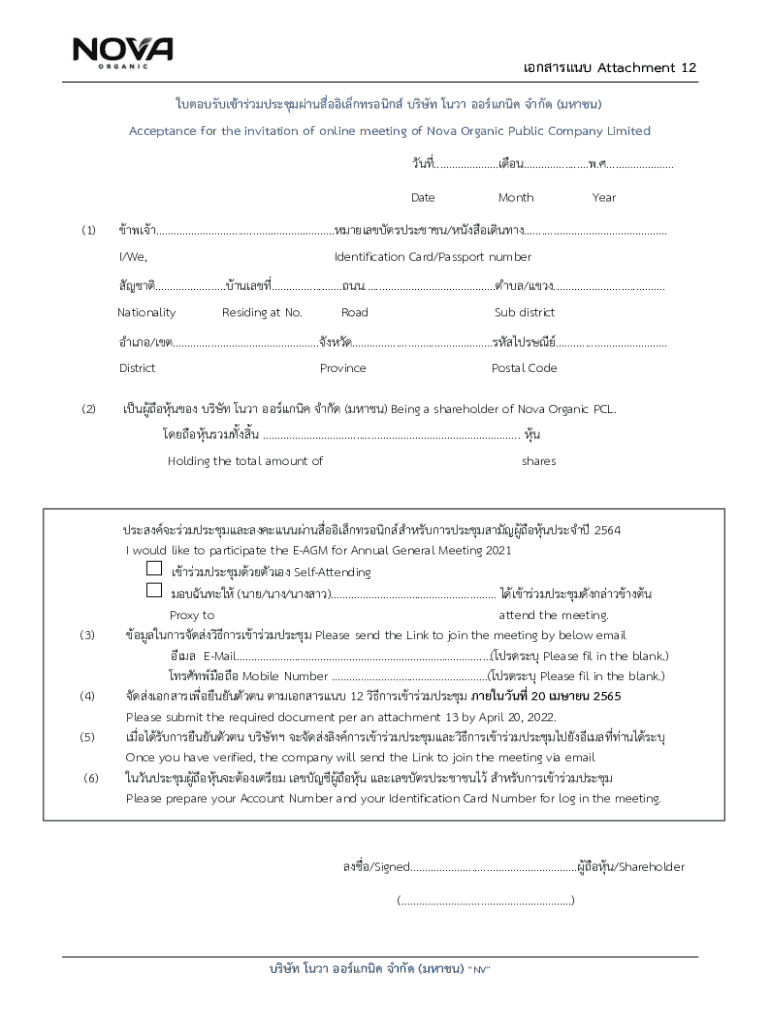
Get the free III CONSENT FORM Study title: Researcher name: ERGO ...
Get, Create, Make and Sign iii consent form study



How to edit iii consent form study online
Uncompromising security for your PDF editing and eSignature needs
How to fill out iii consent form study

How to fill out iii consent form study
Who needs iii consent form study?
Consent Form Study Form: A Comprehensive How-to Guide
Overview of consent form study form
Consent forms are fundamental to the ethical conduct of research studies, serving as documentation that participants understand their role, the study, and any associated risks. The iii consent form study form is specifically designed to ensure this process is clear and compliant with regulatory standards.
The primary aim of the iii consent form is to provide transparency and protect participant rights, which is crucial for fostering trust. Key elements include a clear title, purpose, risks, benefits, and assurances of confidentiality. Additionally, researchers must be aware of regulations set forth by institutional review boards (IRBs) and other governing bodies to ensure that their consent forms meet necessary requirements.
Understanding the types of consent forms
There are various types of consent forms used in research, each tailored to specific purposes and populations. Standard consent forms are the most common, typically consisting of a one-size-fits-all format. However, given the diverse needs of research participants, electronic consent forms (eConsent) have gained traction due to their convenience and accessibility.
eConsent streamlines the process by allowing participants to review and sign documents digitally, which can increase participation rates. When implementing eConsent, consider using user-friendly platforms that enhance the participant experience. Additionally, specialized consent forms are essential for vulnerable populations, such as minors or individuals with cognitive impairments, ensuring they are adequately informed and protected.
Downloading consent form templates
Accessing templates for the iii consent form is made easy through resources like pdfFiller’s library. Users can find a variety of templates to ensure compliance with different regulatory requirements and adapt them to fit specific study parameters.
When downloading a template, following a step-by-step process helps ensure that all required elements are included. After downloading, customize your template by incorporating specific study information and considering language and format that ensures participant comprehension.
Crafting an effective consent form
An effective iii consent form encompasses several key components that communicate vital information to participants. Start with creating a clear title and introduction that captures the essence of your study. The purpose of the study must explicitly outline what is being researched and why the participant's involvement is critical.
Detail the procedures and activities participants will engage in, including the time commitment involved. Clearly outline potential risks and benefits, ensuring to address both physical and emotional facets. Confidentiality is paramount; inform participants how their data will be stored and used, reassuring them about their privacy. Lastly, provide contact information for any queries related to the study.
Interactive tools for form management
Utilizing interactive tools enhances the management of the iii consent form process. pdfFiller offers robust editing features allowing users to make quick changes to PDF content. For instance, users can easily add signatures or eSignatures, making it simple for participants to complete the consent process without cumbersome paperwork.
Collaboration is another key feature. In team-based studies, sharing documents among team members facilitates feedback and ensures that everyone is aligned on the project’s objectives. Tracking changes and comments within the platform also enhances communication and streamlines the revision process.
Considerations for study planning and review
Before launching a study, evaluating the adequacy of your consent form is essential. Engaging with an Institutional Review Board (IRB) helps to ensure compliance with ethical standards and regulatory requirements. Their feedback can be invaluable in refining your forms to meet the highest ethical standards.
Pilot testing the consent forms can also reveal unforeseen challenges and allow for adjustments based on participant feedback. Metrics, such as understanding and retention of information, can provide insight into the effectiveness of consent retrieval.
Implementing the consent process
Outlining the consent process within your study protocols is critical for maintaining clarity and structure. Best practices for obtaining consent emphasize engaging participants, actively discussing the consent form, and ensuring they understand their rights and responsibilities throughout the study.
To ensure unambiguous consent, implement strategies such as summarizing key points of the consent form, inviting questions, and providing multiple opportunities for participants to ask for clarification. This approach fosters a respectful dialogue and ensures that participants feel comfortable and informed.
Managing continuous consent
Continuous consent is vital, particularly as studies evolve or when new information arises that could affect participants. Reconsenting is necessary when significant changes occur, and it's essential to avoid confusion about whether to issue amendments to the existing form or create a new one. Keeping participants informed is crucial to maintaining trust.
Managing withdrawals from studies also necessitates a clear understanding of ongoing consent. Participants should be able to withdraw their consent at any point without repercussions, and this must be clearly communicated within the consent form.
Compliance and ethical considerations in consent
Compliance with regulatory requirements concerning consent is paramount. Researchers must adhere to guidelines set forth by local and international regulatory bodies to protect participant rights. Furthermore, it is essential to maintain stringent ethical standards throughout the research process.
Best practices focus on safeguarding participant rights by providing clear, accessible information about the study and ensuring a thorough understanding before participants agree to take part. In addition, regular ethical reviews and adherence to enhanced standards are vital for maintaining the integrity of the research process.
Navigating the technology of consent
Technology has transformed the consent process, enhancing accessibility and efficiency. Integrating multimedia elements, such as informational videos or FAQs, into your consent process can significantly improve participant understanding of the study and its requirements. Utilizing cloud-based platforms for document management further ensures that documents are easily accessible and securely stored.
Platforms like pdfFiller offer numerous advantages, including enhanced security features and the ability to track document modifications over time, thus streamlining the consent management process while maintaining compliance.
Real-world examples and case studies
Studying successful implementations of the iii consent form can yield valuable insights. For instance, in a recent clinical trial, a well-crafted consent form led to enhanced participant compliance and trust, demonstrating how clarity in communication can significantly impact study outcomes.
On the other hand, examining challenges faced in past studies, such as unclear terminology or insufficient details about risks, highlights the necessity for continual improvements in consent document development. These lessons learned can guide future researchers in creating more effective consent forms.
Additional considerations
When crafting the iii consent form, it’s crucial to be mindful of the diversity within participant populations. Tailoring the form to accommodate cultural differences ensures that all potential participants can engage meaningfully with the content. This cultural competence fosters respect and inclusivity, enhancing participant trust and satisfaction.
Transparency is also a vital principle in the consent process. By openly communicating the study's purpose, potential risks, and the handling of personal information, researchers can cultivate a more approachable and trustworthy relationship with participants.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an electronic signature for the iii consent form study in Chrome?
Can I create an electronic signature for signing my iii consent form study in Gmail?
How do I complete iii consent form study on an iOS device?
What is iii consent form study?
Who is required to file iii consent form study?
How to fill out iii consent form study?
What is the purpose of iii consent form study?
What information must be reported on iii consent form study?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















