Get the free C/O BENITEC BIOPHARMA INC
Get, Create, Make and Sign co benitec biopharma inc



Editing co benitec biopharma inc online
Uncompromising security for your PDF editing and eSignature needs
How to fill out co benitec biopharma inc

How to fill out co benitec biopharma inc
Who needs co benitec biopharma inc?
Comprehensive Guide to the Co Benitec Biopharma Inc Form
Understanding the Co Benitec Biopharma Inc form
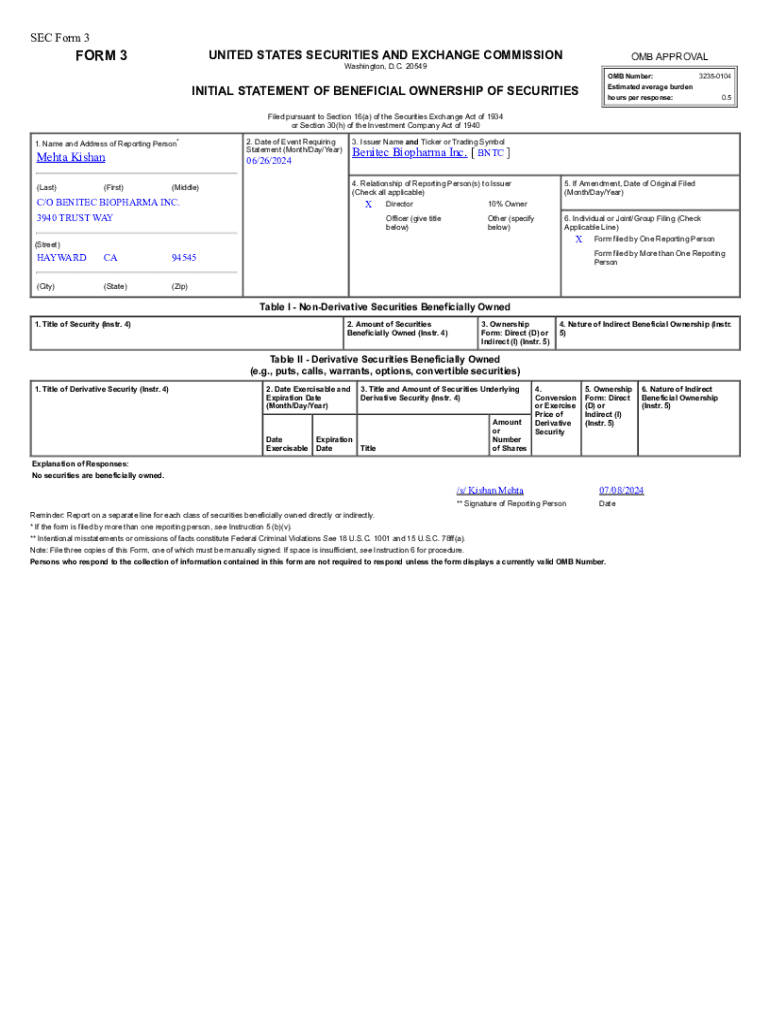
Co Benitec Biopharma Inc is a pioneering biotechnology company focused on developing innovative therapies for unmet medical needs. The company's mission centers around using its proprietary DNA-directed RNA interference (ddRNAi) technology to create effective treatments that can transform healthcare outcomes. Given the complex nature of the biotech industry, understanding the Co Benitec Biopharma Inc form is crucial for various stakeholders, including investors, employees, and regulatory bodies. This form serves as a key documentation piece that signifies compliance, communication, and operational transparency.
Types of forms related to Co Benitec Biopharma Inc
Various forms are associated with Co Benitec Biopharma Inc, each serving distinct purposes essential for different aspects of the company’s operations. Understanding these document types assists stakeholders in navigating through the complexities of biotech regulations and corporate administration effectively.
SEC filings
Biotech companies, like Co Benitec, are required to file several reports with the U.S. Securities and Exchange Commission (SEC). These filings range from quarterly results to more in-depth annual reports. Accurate and timely reporting is crucial to maintain compliance with securities laws and build investor trust.
Corporate governance documentation
Corporate governance documentation outlines the framework within which Co Benitec operates. This includes bylaws, board structure, and policies that govern the organization. Understanding these forms helps clarify the roles and responsibilities of management and the board of directors, ensuring accountability and ethical conduct.
Financial reports
Financial reports are paramount for stakeholder communication. They encompass detailed financial health narratives of Co Benitec, addressing revenue, expenses, and strategic direction. These documents are vital for evaluating company stability and investment potential.
Steps to access and fill out the Co Benitec Biopharma Inc form
Filling out the Co Benitec Biopharma Inc form involves several critical steps that guarantee accuracy and compliance, ensuring that stakeholders are properly informed and involved in the process.
Locating the official form
To access the Co Benitec Biopharma Inc form, it can be found on platforms like pdfFiller, which provides a user-friendly experience for document management. Additionally, official channels such as the SEC's website provide access to necessary filings and related documents.
Essential information required
When filling out the Co Benitec Biopharma Inc form, ensure you have the following information ready:
How to fill out the form effectively
Filling out the form correctly is critical. Here are some tips to ensure accuracy and completeness:
Editing and managing the Co Benitec Biopharma Inc form
Once the Co Benitec Biopharma Inc form is filled out, managing and editing becomes essential for documentation accuracy and clarity. With tools from pdfFiller, this process can be simplified considerably.
Leveraging pdfFiller tools
pdfFiller offers a suite of interactive tools that make editing intuitive. Features like highlighting, commenting, and revision tracking streamline the revision process, allowing users to make necessary adjustments confidently.
Collaborative features
The platform allows multiple team members to collaborate on the Co Benitec Biopharma Inc form. Inviting colleagues to review and give feedback not only enhances the quality of the document but also fosters a culture of collaboration. Furthermore, tracking changes and version history maintains transparency throughout the editing process.
Signing the Co Benitec Biopharma Inc form
Once all necessary information has been entered and edits have been made, the next step is signing the Co Benitec Biopharma Inc form. This process is essential for formalizing the completion of the document, ensuring that all parties agree to the terms laid out.
eSignature solutions
Using pdfFiller’s eSignature capabilities brings convenience and efficiency in the signing process. The platform allows users to sign documents electronically, ensuring a streamlined approach to completing the Co Benitec Biopharma Inc form.
Legal considerations
Understanding the legality of digital signatures versus traditional signing is crucial in the context of biotech documentation. In most jurisdictions, eSignatures hold the same legal weight as handwritten signatures when executed properly, making it a viable option for Co Benitec Biopharma Inc.
Submitting the completed Co Benitec Biopharma Inc form
After signing, the Co Benitec Biopharma Inc form must be submitted. The process of submission is critical to avoid delays and ensure compliance with regulatory requirements.
Submission channels
There are various methods available for submitting the Co Benitec Biopharma Inc form. Your submission method may depend on the specific requirements outlined in the form.
What to expect after submission
Once submitted, stakeholders can expect a verification process. This may include acknowledgment of receipt and a timeframe for potential follow-ups. It’s important to keep records of submissions to track progress and ensure compliance.
Frequently asked questions about the Co Benitec Biopharma Inc form
Navigating the requirements and processes tied to the Co Benitec Biopharma Inc form can raise questions. Here are some commonly asked queries that individuals may encounter.
For troubleshooting any common issues, it is advisable to consult the guidelines provided on pdfFiller or reach out for support for assistance.
Additional tools and resources for document management
Using the right tools is essential for effective document management in biotechnology settings. pdfFiller provides robust solutions for document creation, editing, and storage, minimizing the risks associated with mismanagement.
pdfFiller's comprehensive document solutions
Features offered by pdfFiller empower users to seamlessly create, manage, and store documents in a secure environment, enhancing productivity and collaboration. Capabilities include advanced search functionality, easy sharing options, and integration with existing systems.
Best practices for document handling in biotech firms
Maintaining compliance and accuracy in all documentation is especially critical within biotech firms like Co Benitec. Adhering to best practices ensures that data integrity is upheld while facilitating regulatory compliance. Practices include:
Future updates and regulatory changes impacting the Co Benitec Biopharma Inc form
The biotechnology industry is subject to rapid changes in regulations, which can significantly impact documentation processes, including the Co Benitec Biopharma Inc form. Staying informed about these updates is vital for compliance and operational efficiency.
Anticipated changes may include tighter scrutiny on clinical trial reporting, data management regulations, or modifications to funding disclosures. It’s advisable for stakeholders to keep abreast of evolving regulatory landscapes to ensure documentation remains current and compliant.
Establishing a system for monitoring regulatory changes can also enhance organizational readiness, where adjustments can be made proactively rather than reactively.
An effective way to stay informed is through industry newsletters, webinars, or dedicated regulatory bodies' communications, which provide valuable insights into upcoming changes.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an electronic signature for the co benitec biopharma inc in Chrome?
How do I fill out co benitec biopharma inc using my mobile device?
How do I complete co benitec biopharma inc on an iOS device?
What is co benitec biopharma inc?
Who is required to file co benitec biopharma inc?
How to fill out co benitec biopharma inc?
What is the purpose of co benitec biopharma inc?
What information must be reported on co benitec biopharma inc?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

























