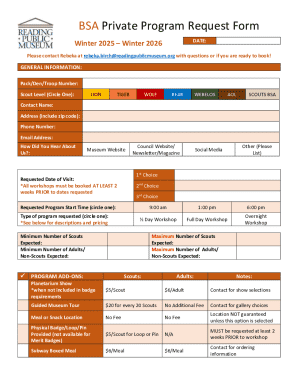
Get the free Trial Results :: Huntington, MA July NW3, Elite Division ...
Get, Create, Make and Sign trial results huntington ma



How to edit trial results huntington ma online
Uncompromising security for your PDF editing and eSignature needs
How to fill out trial results huntington ma

How to fill out trial results huntington ma
Who needs trial results huntington ma?
Trial results Huntington MA form: A comprehensive how-to guide
Understanding the trial results form
The trial results form is a critical document in clinical research, particularly for studies related to Huntington's disease. This form is designed to summarize the findings of a clinical trial, encompassing both quantitative data and qualitative observations. Its purpose extends beyond mere compliance; it serves as a record that can influence future research directions, treatment decisions, and patient care strategies.
This form is necessary for researchers, clinicians, and institutions involved in trials. Anyone responsible for reporting trial outcomes—whether a principal investigator, a clinical research coordinator, or statistical analysts—needs to complete this form. Moreover, understanding trial results helps ensure transparency and accountability in research, critical for ethical compliance and public trust.
Key definitions related to the trial results
Trial results within the context of Huntington's disease can refer to various outcomes, including symptom improvement, cognitive function changes, and overall quality of life metrics. Key medical terminologies such as 'endpoints,' 'adverse events,' and 'randomization' should be clearly understood by those filling out the form. Each term carries weight in draft documentation, influencing interpretations and conclusions drawn from the data.
Filling out the trial results form
Completing the trial results form is a meticulous process requiring attention to detail. Start by gathering all necessary information before diving into filling the form. This ensures that the requisite data is readily available and minimizes the risk of missing critical information.
Common mistakes to avoid
When completing the trial results form, some common pitfalls may include submitting incomplete information, such as missing patient identifiers or neglecting to include essential data points. Additionally, misinterpreting data can lead to erroneous conclusions, undermining the integrity of the research. It’s critical to double-check that all sections of the form are thoroughly completed and that information is presented clearly.
Editing and reviewing the form
Editing and reviewing the trial results form is just as important as filling it out correctly. Utilizing pdfFiller provides an efficient way to edit documents, offering tools for corrections and adjustments. Features such as highlighting, comments, and suggestions can facilitate effective team collaboration, ensuring that every member’s input is considered.
Signing the trial results form
The importance of electronic signatures (eSignatures) cannot be overstated in the realm of clinical trials. eSigning the trial results form upholds compliance with regulatory standards and simplifies the review process. The process generally involves verifying identity to ensure security before a signature can be applied.
Alternatives to eSigning if necessary
In scenarios where eSigning is not feasible, traditional methods of signing, such as printing the document, signing it manually, and then rescanning, may be employed. However, using eSigning is generally more efficient, offering better tracking and more straightforward management.
Managing your trial results form
Once your trial results form is complete and signed, effective document management becomes essential. pdfFiller's cloud-based platform enables users to store documents securely, organize them intuitively, and access them anytime, anywhere. This is particularly valuable for teams working remotely or in different locations.
Security measures to protect sensitive information
Being in the healthcare realm, protecting sensitive information is paramount. This includes implementing robust security measures when sharing the trial results form. pdfFiller offers encryption standards and secure sharing options, ensuring that only authorized individuals can access the data.
Interactive tools and features
The interactive features of pdfFiller enhance the document completion experience. Access to customizable templates for forms allows users to begin with a tailored approach rather than starting from scratch, saving time and ensuring compliance with expected standards.
Utilizing analytics features
Using analytics features allows for better insights into form usage and submission trends, which can guide future research strategies. Collecting user feedback also helps in iterating and improving templates, ensuring they remain relevant and user-friendly.
Common FAQs about the trial results form
Questions often arise during the process of completing the trial results form. Frequently asked questions typically focus on technical difficulties encountered while filling out or submitting the form, along with queries regarding the timeline for updates. Being prepared with answers to these questions can help streamline the process.
Case studies: Successful use of trial results form
Real-life examples of completed forms highlight varied scenarios in Huntington’s disease research and the diversity of outcomes that can emerge from clinical trials. Understanding these case studies provides valuable insights into how accurately filled trial results forms can provide clarity and assist in further studies.
Staying informed on trial results
Continuous learning is crucial in the evolving field of clinical trials. Keeping up-to-date with ongoing research related to Huntington's disease helps researchers and healthcare professionals stay informed about the latest findings and methodologies.
Key takeaways
Utilizing the trial results Huntington MA form effectively contributes to advancing clinical research on Huntington’s disease. pdfFiller offers comprehensive, cloud-based solutions for keeping this vital component organized, signed, and accessible. A commitment to accurate documentation and effective reporting ensures the integrity of the study while enhancing the success of clinical trials moving forward.
By harnessing the tools available through pdfFiller, users can streamline the paperwork associated with clinical trials and focus more on the results that can drive innovations in patient care and treatment.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify trial results huntington ma without leaving Google Drive?
How do I execute trial results huntington ma online?
How do I edit trial results huntington ma straight from my smartphone?
What is trial results huntington ma?
Who is required to file trial results huntington ma?
How to fill out trial results huntington ma?
What is the purpose of trial results huntington ma?
What information must be reported on trial results huntington ma?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.