Get the free EYEGATE PHARMACEUTICALS INC EYEG
Get, Create, Make and Sign eyegate pharmaceuticals inc eyeg



How to edit eyegate pharmaceuticals inc eyeg online
Uncompromising security for your PDF editing and eSignature needs
How to fill out eyegate pharmaceuticals inc eyeg

How to fill out eyegate pharmaceuticals inc eyeg
Who needs eyegate pharmaceuticals inc eyeg?
Eyegate Pharmaceuticals Inc Eyeg Form - How-to Guide
Understanding the Eyegate Pharmaceuticals Inc Eyeg Form
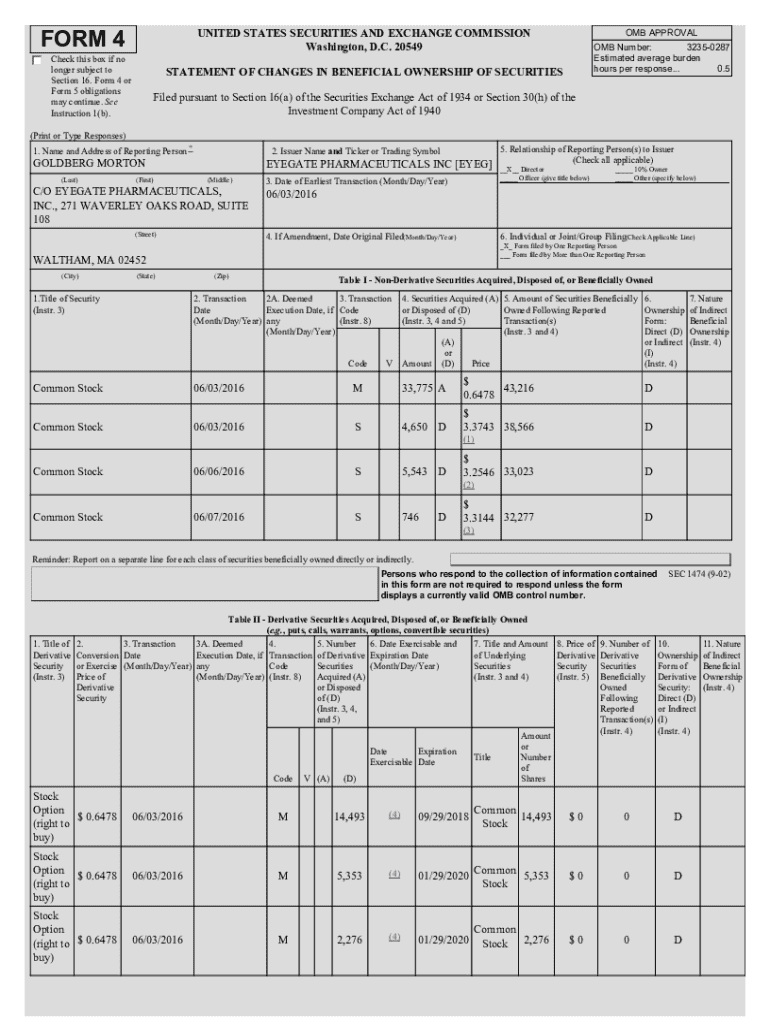
The Eyegate Form is a vital documentation tool utilized by Eyegate Pharmaceuticals Inc to facilitate the efficient management of clinical trial data and compliance. This form serves as a comprehensive record that supports the integrity of the pharmaceutical development process, ensuring that all necessary information is captured meticulously. As the pharmaceutical industry increasingly emphasizes transparency and accuracy, the importance of such documentation cannot be overstated.
Key stakeholders involved in the usage of the Eyeg Form include clinical researchers, regulatory professionals, and quality assurance teams. These parties rely on the form to communicate essential information about clinical trials, patient data, and regulatory compliance. Consequently, the Eyeg Form plays an instrumental role in streamlining communication between these stakeholders, ultimately enhancing the integrity of clinical trials and expediting the development of innovative therapies.
At Eyegate Pharmaceuticals, the Eyeg Form is integral to the company’s clinical development strategy. For instance, the ongoing projects involve investigations into novel drug delivery systems for ocular diseases, where capturing accurate data through the Eyeg Form is essential for regulatory submissions and study assessments.
Detailed breakdown of the Eyeg Form
Understanding the structure of the Eyeg Form is crucial for its effective completion. The form typically consists of several key sections, each designed to capture specific types of information necessary for comprehensive documentation. These sections include fields for patient demographics, safety information, trial details, and investigator credentials.
Each section of the Eyeg Form carries significance in the documentation process. For instance, the Safety Information section is critical for meeting regulatory standards, while the Patient Demographics section ensures holistic patient representation in research findings.
Users may encounter terminology that can be confusing when completing the Eyeg Form. Commonly used terms include 'adverse event,' 'informed consent,' and 'protocol deviation.' It’s essential to familiarize oneself with these terms to enhance understanding and compliance when filling out the form.
Step-by-step guide to completing the Eyeg Form
Completing the Eyeg Form requires careful preparation and attention to detail. Let’s break down the process into systematic steps:
By following these steps, users can improve both the accuracy and timeliness of their submissions.
Leveraging pdfFiller for enhanced form management
pdfFiller offers a plethora of interactive tools that can significantly aid in managing the Eyeg Form. With features designed to streamline the documentation process, users can edit, sign, and store forms seamlessly. The platform provides an intuitive interface that simplifies access to necessary features, allowing users to focus more on content rather than technical hurdles.
One remarkable aspect of pdfFiller is its collaborative options, which enable multiple team members to work on the Eyeg Form simultaneously. This collaboration ensures transparency and enhances productivity within teams, as everyone can provide input and ensure that the form reflects comprehensive data input.
Additionally, the cloud-based document management system of pdfFiller affords users the advantage of accessing their documents from anywhere, at any time. This accessibility ensures that users can make edits and updates on-the-go, enhancing their ability to stay organized and compliant.
Addressing common challenges and FAQs
When tackling the Eyeg Form, users often have questions about specific aspects of the form completion and submission processes. Common FAQs include ‘What should I do if I make an error after submitting the form?’ and ‘How often does the Eyeg Form's content change?’ To address such queries, it’s crucial to maintain open communication with regulatory bodies and stay updated on any forthcoming changes in documentation procedures.
Best practices for ensuring compliance and efficiency include maintaining organized electronic files and scheduling regular reviews to ensure that all team members are aligned on updates or changes to the form.
Continuous updates and monitoring
Staying informed about changes to the Eyeg Form is crucial for maintaining compliance and ensuring that the most current information is leveraged in trials. Eyegate Pharmaceuticals regularly updates stakeholders, so subscribing to their announcements or newsletters is highly recommended to keep abreast of the latest developments.
Regularly reviewing documentation procedures also plays a fundamental role in ensuring compliance. Companies must set internal audits to reassess how the form is being utilized and make necessary adjustments based on regulatory updates or internal quality controls.
Case studies and user experiences
Real-world examples highlight how effective usage of the Eyeg Form can lead to successful clinical outcomes. For instance, a notable case involved a research team that streamlined their documentation process using pdfFiller, resulting in a faster turnaround for trial approvals and better resource allocation during clinical studies.
User testimonials from pdfFiller clients within the pharmaceutical sector consistently praise the platform for enhancing accuracy in form management and significantly reducing time spent on document handling. These experiences underscore the importance of integrating effective tools like pdfFiller in clinical research endeavors.
Engaging with Eyegate Pharmaceuticals
To remain updated on Eyegate Pharmaceuticals’ developments, individuals can subscribe to newsletters, follow their official social media accounts, or engage with their website for the latest drug developments and updates regarding the Eyeg Form. Following these channels will ensure that users never miss essential updates that may affect their work.
Connecting with the Eyegate community is also beneficial, as forums and discussion boards allow professionals to share insights, ask questions, and gain advice from peers who are navigating similar challenges and opportunities related to the Eyeg Form.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I get eyegate pharmaceuticals inc eyeg?
Can I create an electronic signature for the eyegate pharmaceuticals inc eyeg in Chrome?
Can I edit eyegate pharmaceuticals inc eyeg on an Android device?
What is eyegate pharmaceuticals inc eyeg?
Who is required to file eyegate pharmaceuticals inc eyeg?
How to fill out eyegate pharmaceuticals inc eyeg?
What is the purpose of eyegate pharmaceuticals inc eyeg?
What information must be reported on eyegate pharmaceuticals inc eyeg?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















