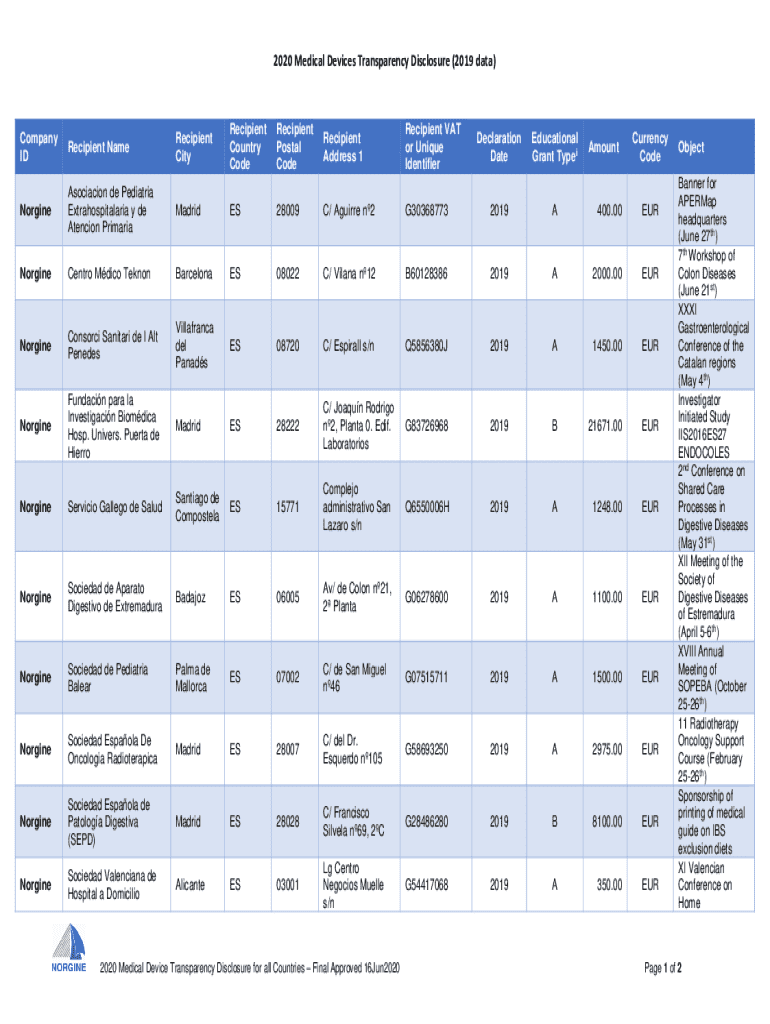
Get the free 2020 Medical Devices Transparency Disclosure (2019 data)
Get, Create, Make and Sign 2020 medical devices transparency



How to edit 2020 medical devices transparency online
Uncompromising security for your PDF editing and eSignature needs
How to fill out 2020 medical devices transparency

How to fill out 2020 medical devices transparency
Who needs 2020 medical devices transparency?
2020 Medical Devices Transparency Form: A Comprehensive Guide
Understanding the 2020 medical devices transparency form
Transparency in medical devices is critical for fostering trust between manufacturers, healthcare providers, and patients. As healthcare evolves and patients become more informed consumers, the importance of knowing their medical devices' details cannot be overstated. The 2020 medical devices transparency form emerges within this context, establishing a standardized way to disclose vital product information.
Regulatory bodies, particularly the FDA in the United States and the EMA in the European Union, mandate increased transparency in medical device reporting. These requirements reflect a broader trend in healthcare, emphasizing the need for openness around clinical data, performance metrics, and potential conflicts of interest that could affect patient care.
Purpose of the 2020 medical devices transparency form
The primary aim of the 2020 medical devices transparency form is to consolidate extensive information about medical devices in a single document. By detailing product information and disclosures, the form acts as a vital resource for healthcare providers and patients alike, enabling better decision-making associated with medical treatments.
Moreover, the increased clarity provided by the form can significantly enhance trust among stakeholders. Manufacturers can demonstrate their commitment to transparency, while healthcare providers gain access to crucial data that can support clinical decisions. This synergy is essential in building a healthcare landscape where patients feel safer and more informed.
Key components of the 2020 medical devices transparency form
To effectively communicate essential information, the 2020 medical devices transparency form consists of several key components. Each section is designed to guide the user in presenting the necessary details to fulfill compliance requirements thoroughly.
Documentation and evidence
Completing the 2020 medical devices transparency form necessitates comprehensive documentation that supports the information disclosed. This ensures that the submission complies with the stringent requirements set by regulatory authorities.
Types of documents to accompany the form may include clinical study reports, risk management files, and evidence of conformity with relevant manufacturing standards. By carefully gathering and organizing these documents, manufacturers can avoid compliance issues and promote a smoother review process.
Step-by-step guide to completing the form
Successfully completing the 2020 medical devices transparency form involves a strategic approach. Proper preparation and thorough understanding of each section of the form are crucial for avoiding delays or rejections in the submission process.
Common pitfalls include failing to provide complete information or neglecting to fully disclose financial relationships or conflicts of interest. To avoid these issues, thorough proofreading and ensuring that all fields are filled accurately is essential.
Tools and resources for efficient form management
Utilizing advanced tools like pdfFiller can greatly enhance your experience with the 2020 medical devices transparency form. The platform offers features that simplify the form editing and completion process, ensuring users can easily make necessary adjustments.
Ensuring compliance and understanding regulations
Navigating the landscape of medical device regulations is critical for compliance. Key regulatory bodies like the FDA in the U.S. and the EMA in Europe hold the responsibility for establishing guidelines that affect medical device submissions and transparency.
Understanding Good Manufacturing Practices (GMP) is fundamental for any manufacturer seeking compliance. The transparency form serves as an extension of these practices, where demonstrated adherence to GMP can facilitate market access and promote trustworthiness in products.
Navigating international compliance standards can be challenging due to variations between regions. It’s essential for manufacturers to stay informed about both regional and global trends in medical device regulation.
Post-submission: What happens next?
Once the 2020 medical devices transparency form is submitted, it enters a review process governed by regulatory authorities. Understanding what entails this process is key for managing expectations regarding timelines and outcomes.
Real-world impact of medical devices transparency
The adoption of the 2020 medical devices transparency form has led to significant benefits within the medical industry. Case studies illustrate how major manufacturers have successfully harnessed transparency to bolster patient confidence and secure a competitive advantage in the marketplace.
For instance, companies that openly disclose clinical results and safety information tend to establish stronger relationships with healthcare providers and patients. This transparency not only enhances safety but also cultivates a sense of obligation among manufacturers to uphold high standards of care.
Future of medical devices transparency
As the medical device industry progresses, we can anticipate upcoming changes in regulations and standards that will further shape transparency efforts. Stakeholders should remain proactive in adapting to these changes to ensure ongoing compliance.
Additional insights and interactive resources
For those looking to deepen their understanding of the 2020 medical devices transparency form, numerous resources provide valuable insights. Industry reports and white papers often analyze the trends and best practices surrounding transparency in medical devices.
Engaging with the pdfFiller community
Users of pdfFiller have found the platform indispensable for managing their documentation needs. Testimonials highlight the efficiency and ease of use when editing, signing, and collaborating on documents like the 2020 medical devices transparency form.






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find 2020 medical devices transparency?
Can I create an electronic signature for signing my 2020 medical devices transparency in Gmail?
How do I edit 2020 medical devices transparency on an iOS device?
What is 2020 medical devices transparency?
Who is required to file 2020 medical devices transparency?
How to fill out 2020 medical devices transparency?
What is the purpose of 2020 medical devices transparency?
What information must be reported on 2020 medical devices transparency?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.






















