Get the free MARKETING AUTHORISATION NUMBER SYSTEM
Show details
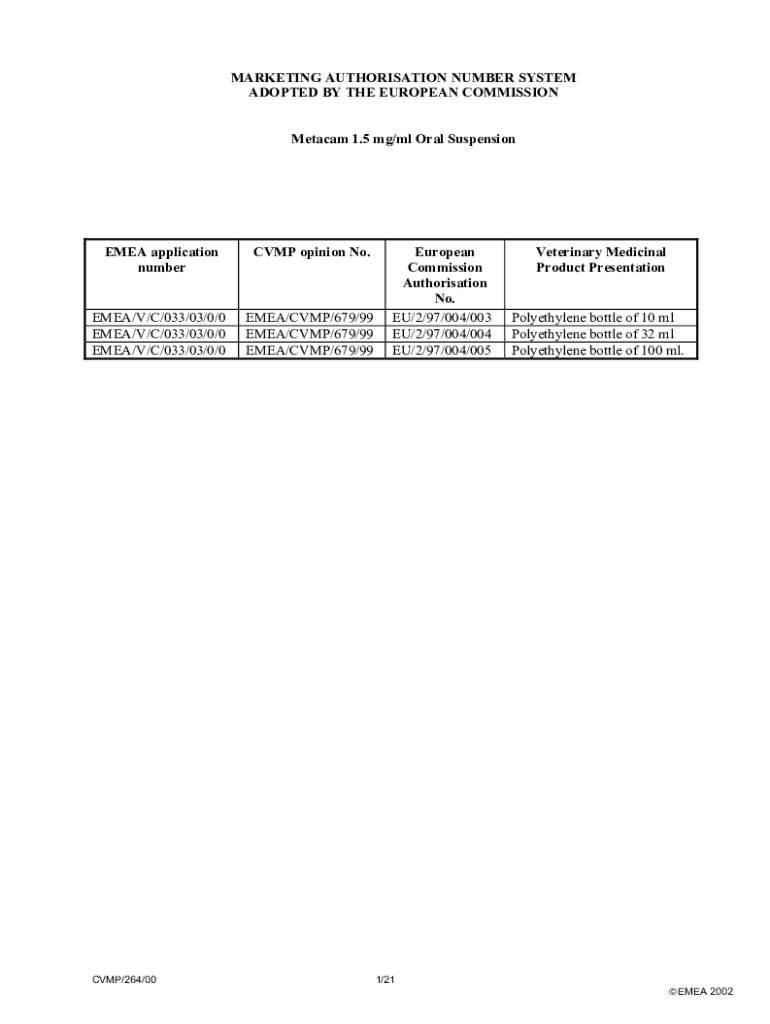
MARKETING AUTHORISATION NUMBER SYSTEM ADOPTED BY THE EUROPEAN COMMISSION 1.5 mg/ml Oral SuspensionEMEA application numberCVMP opinion No.EMEA/V/C/033/03/0/0 EMEA/V/C/033/03/0/0 EMEA/V/C/033/03/0/0EMEA/CVMP/679/99 EMEA/CVMP/679/99 EMEA/CVMP/679/99CVMP/264/00European Commission Authorisation No. EU/2/97/004/003 EU/2/97/004/004 EU/2/97/004/005Veterinary Medicinal Product PresentationPolyethylene bottle of 10 ml Polyethylene bottle of 32 ml Polyethylene bottle of 100 ml.1/21 EMEA
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign marketing authorisation number system

Edit your marketing authorisation number system form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your marketing authorisation number system form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit marketing authorisation number system online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit marketing authorisation number system. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out marketing authorisation number system

How to fill out marketing authorisation number system
01
Step 1: Gather all necessary documentation related to the product that requires authorization.
02
Step 2: Ensure your product complies with the regulatory standards applicable in your jurisdiction.
03
Step 3: Complete the application form required for the marketing authorisation number system.
04
Step 4: Submit the required documents along with the application form to the relevant regulatory authority.
05
Step 5: Pay any applicable fees associated with the application process.
06
Step 6: Await confirmation of receipt and processing of your application from the regulatory authority.
07
Step 7: Respond promptly to any queries from the regulatory authority during the review process.
08
Step 8: Once approved, receive your marketing authorisation number and ensure it is displayed on your product packaging.
Who needs marketing authorisation number system?
01
Pharmaceutical companies seeking to market new drugs.
02
Manufacturers of medical devices intending to sell their products.
03
Biotechnology firms looking to commercialize biological products.
04
Importers and distributors of regulated health products.
05
Healthcare organizations planning to introduce new treatments or therapies.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit marketing authorisation number system from Google Drive?
You can quickly improve your document management and form preparation by integrating pdfFiller with Google Docs so that you can create, edit and sign documents directly from your Google Drive. The add-on enables you to transform your marketing authorisation number system into a dynamic fillable form that you can manage and eSign from any internet-connected device.
Where do I find marketing authorisation number system?
With pdfFiller, an all-in-one online tool for professional document management, it's easy to fill out documents. Over 25 million fillable forms are available on our website, and you can find the marketing authorisation number system in a matter of seconds. Open it right away and start making it your own with help from advanced editing tools.
How do I edit marketing authorisation number system on an Android device?
The pdfFiller app for Android allows you to edit PDF files like marketing authorisation number system. Mobile document editing, signing, and sending. Install the app to ease document management anywhere.
What is marketing authorisation number system?
The marketing authorisation number system is a regulatory framework used to approve and monitor the sale of pharmaceutical products, ensuring they meet necessary safety, efficacy, and quality standards before being marketed to the public.
Who is required to file marketing authorisation number system?
Manufacturers and distributors of pharmaceutical products are required to file for a marketing authorisation number, which includes companies seeking to introduce new drugs or change existing drug formulations.
How to fill out marketing authorisation number system?
To fill out the marketing authorisation number application, applicants must complete a detailed form that includes product information, clinical trial data, labeling, and safety information, and submit it to the relevant regulatory authority.
What is the purpose of marketing authorisation number system?
The purpose of the marketing authorisation number system is to ensure that only safe and effective pharmaceutical products reach the market, providing oversight and regulation to protect public health.
What information must be reported on marketing authorisation number system?
Information that must be reported includes the product’s active ingredients, manufacturing processes, clinical trial results, proposed labeling, and any safety or adverse event data associated with the product.
Fill out your marketing authorisation number system online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Marketing Authorisation Number System is not the form you're looking for?Search for another form here.
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.