Get the free Investigator Initiated Clinical Study Agreement between the Research Foundation for ...
Show details
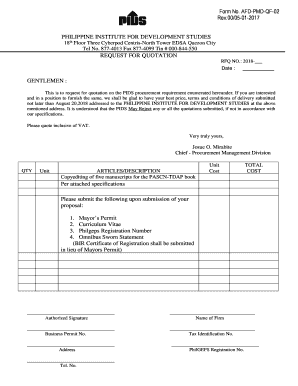
This is a sample Investigator Initiated Clinical Study Agreement between the Research Foundation for the State and the Sponsor. A clinical trial agreement is a standard legal agreement between
We are not affiliated with any brand or entity on this form
Why choose pdfFiller for your legal forms?
All-in-one solution
pdfFiller offers a PDF editor, eSignatures, file sharing, collaboration tools, and secure storage—all in one place.
Easy to use
pdfFiller is simple, cloud-based, has a mobile app, and requires no downloads or a steep learning curve.
Secure and compliant
With encryption, user authentication, and certifications like HIPAA, SOC 2 Type II, and PCI DSS, pdfFiller keeps sensitive legal forms secure.

What is investigator initiated clinical study
An investigator initiated clinical study is a research trial led by a principal investigator to explore the efficacy and safety of a specific intervention or treatment, independent of external sponsorship.
pdfFiller scores top ratings on review platforms




Great, easy to use and fantastic customer service.
Life safer in completing forms for the State of Maryland.
I HAVE JUST BEEN USING A WEEK BUT IT HAS HELPED ME SO MUCH! EASY TO USE! AND VERY PROFESSIONAL
I have just submitted an application form to the District office. It was very neat as it was the only one filled in electronically.
It works, which is more than I can say of anything else I have tried, and it is easy to work, too.
I run a business, and sometimes PDF Filler is the perfect solution to fill that gap in my standard software. Works great!
Who needs investigator initiated clinical study?
Explore how professionals across industries use pdfFiller.
Comprehensive Guide to Filling Out the Investigator-Initiated Clinical Study Form
How does an investigator-initiated clinical study agreement work?
An Investigator-Initiated Clinical Study Agreement (IICSA) is crucial for defining the responsibilities and expectations of the parties involved in a clinical study. Understanding its components and significance lays the groundwork for successful research collaboration.
-
Investigator-initiated studies empower researchers to explore innovative therapies, catering to unmet medical needs.
-
It typically includes terms related to funding, data sharing, and intellectual property rights, ensuring clarity in research duties.
-
A meticulously drafted IICSA fosters trust and facilitates smooth collaboration between investigators and sponsors.
What are the steps to completing the agreement?
Completing the IICSA involves careful attention to detail to ensure accuracy. Each section of the form must reflect the collaborative spirit and specific needs of the study being undertaken.
-
Clearly delineate the roles of the institution, principal investigator, and sponsor for accountability.
-
Accurate documentation of the agreement’s initiation is essential, as it impacts project timelines.
-
Articulate the study protocol comprehensively to avoid ambiguity and ensure stakeholder alignment.
What essential clauses should be included and understood?
Certain clauses within the IICSA carry significant legal and operational implications that both parties must understand fully.
-
'WHEREAS' clauses articulate the background and context, providing legal weight to the agreement.
-
Highlighting benefits reinforces partnership integrity and encourages shared commitment to the research goals.
-
Both parties must be aware of their financial and operational responsibilities to avoid future disputes.
How can you edit and sign the form using pdfFiller?
pdfFiller offers an intuitive platform for editing and signing the investigator-initiated clinical study form, streamlining the documentation process for users.
-
Users can customize forms with text fields, checkboxes, and dropdowns, enhancing clarity and utility.
-
The secure eSign feature enables quick signing, thus accelerating the overall agreement process.
-
Real-time collaboration helps teams make swift amendments, improving workflow efficiency.
What compliance measures are vital in clinical research?
Adhering to compliance is not just a legal requirement but a cornerstone of upholding ethical standards in clinical research.
-
Familiarity with laws and guidelines governing clinical studies is essential for investigator and sponsor accountability.
-
Following Good Clinical Practice (GCP) ensures the safety, integrity, and efficacy of the research.
-
Compliance considerations may vary; for instance, studies conducted in the U.S. necessitate adherence to FDA regulations.
How do you insert a protocol title and attachments?
Attaching the study protocol is a critical component of the IICSA, ensuring that all parties are aligned on objectives.
-
The protocol clarifies study methodologies, making it indispensable for regulatory review.
-
The protocol title should precisely reflect the study’s objective to aid in identification during regulatory reviews.
-
pdfFiller allows users to seamlessly add attachments to the IICSA, aligning all documents in one place.
What are the best practices for document management post-completion?
Effective document management strategies are essential for maintaining organized and accessible research documentation after completion.
-
Use cloud-based solutions like pdfFiller to ensure high security and easy access to completed agreements.
-
Implement a system for logging changes and revisions to ensure clarity of the document's history.
-
Utilizing collaborative tools aids in efficient updates and smoother transitions during follow-up studies.
Why is it important to understand key terminology?
Familiarity with key terms is foundational for both investigators and sponsors, as it fosters clearer communication and understanding in clinical research.
-
Understanding terms such as Sponsor, Investigator, and Protocol is crucial for accurate documentation.
-
Being well-versed in clinical research terminology significantly aids in the completion process and improves collaboration.
-
Access to comprehensive glossaries can act as a quick reference for teams dealing with complex terminology.
How to fill out the investigator initiated clinical study
-
1.Ensure you have a clear hypothesis or research question that your study will address.
-
2.Gather all necessary regulatory documents, including ethical approval from an institutional review board.
-
3.Log into pdfFiller and upload your previously designed study protocol template or start a new document.
-
4.Fill in the title of the study, principal investigator details, and all relevant study information including aims, methodology, and expected outcomes.
-
5.Include participant eligibility criteria, recruitment strategies, and data collection methods in the designated sections.
-
6.Provide information on funding sources, if applicable, and budget considerations for the study.
-
7.Review the filled document for any errors or missing information and make necessary corrections.
-
8.Once finalized, save the document as a PDF and share with all stakeholders for their feedback.
-
9.Incorporate any required revisions and finalize the document for submission according to regulatory requirements.
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.