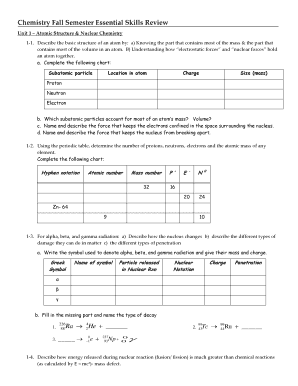
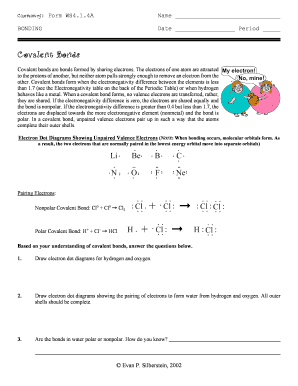
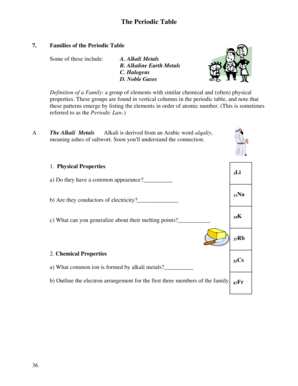
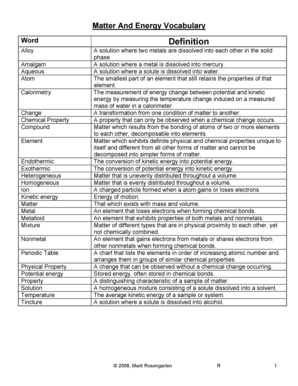
Cl Electronegativity
What is cl electronegativity?
Cl electronegativity refers to the electronegativity value of the chemical element chlorine (Cl). Electronegativity is a measure of an atom's ability to attract shared electrons in a chemical bond. In the case of chlorine, it has a relatively high electronegativity compared to other elements.
What are the types of cl electronegativity?
There are two types of cl electronegativity: 1. Pauling electronegativity: This is a widely used scale for measuring electronegativity and was developed by Linus Pauling. It assigns numeric values to elements based on their electronegativity, with higher values indicating higher electronegativity. Chlorine has a Pauling electronegativity value of 3.16. 2. Mulliken electronegativity: This scale takes into account the average electron affinity and ionization potential of an element. Chlorine has a Mulliken electronegativity value of 3.61.
How to complete cl electronegativity
To complete cl electronegativity, follow these steps:
pdfFiller empowers users to create, edit, and share documents online. Offering unlimited fillable templates and powerful editing tools, pdfFiller is the only PDF editor users need to get their documents done.