Analyze Required Field Certificate For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
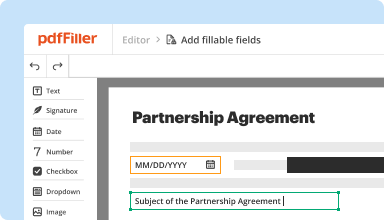
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
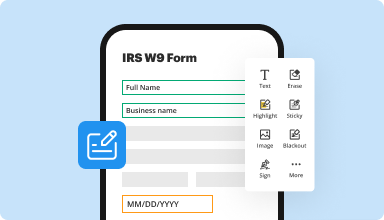
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
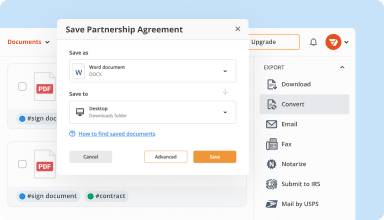
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
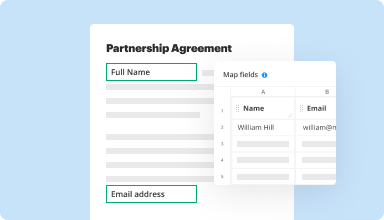
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
this program is the answer to all who work with osha 300 log; thank you so much. Mahalo, and Aloha.
Kalani Whitford / Safety Officer
National Fire Protection Inc.
2015-04-08
Good service. Can be a little difficult to line up the text in the correct spot. Would be good if you could easily move the text box up and down a little to line things up.
2019-08-30
The best PDF converter
Excellent.
My current job is as freelance translator, and sometimes my customers send me the documents in PDF. for me, it's easier to work with WORD format.
You can convert any type of file into editable one.
In case of scanned PDF, it doesn't work.
2019-03-12
Once I figured out the system it was…
Once I figured out the system it was not difficult for me to complete this process. Thank You for streamlining this process.
2023-11-03
Just getting started. Signed up a few months ago but it is only now that I am getting around to cleaning up my forms for a virtual behavioral medicine practice
2021-10-23
best customer service I've experienced
Software works good, had no problem at all. The customer service was top notch. They helped resolve my account problems within the first hour I sent out the form. Polite and efficient. The excellent service provided was the last and fith star.
2021-10-21
This is a wonderful software to use
This is a wonderful software to use. No more paper-printing and scanning in. I just do everything electronically, it saves the environment as well as a lot of time.
2021-06-26
I reviewed this software for my company…
I reviewed this software for my company (we historically use another product) for function and usability. It is significantly more user friendly than the others that do similar functions. What makes this a good decision is the cost and the support. It costs a third of the software we currently use and when I experienced a problem (on the weekend) I had (professional and personal) support online in less than a minute. Very satisfied.
2021-01-09
I needed to use the service for an application! It was only needed once. The day I was going to cancel it due to no longer needing the service the money had already come out of my account. They immediately refunded it and even offered a discount if I wanted to continue using it. I highly recommend it was an easy to use service with many benefits packed in. Customer support replies quickly and they are very professional.
2020-10-15
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
What is a certificate of analysis requirements?
A Certificate of Analysis refers to an authenticated document that is issued by Illumine's Quality Assurance Department that ascertains that a product has met its predetermined product release specification(s) and quality. What information is provided on a Certificate of Analysis? ... Specifications or Requirements.
What is in a certificate of analysis?
Certificates of Analysis. A Certificate of Analysis is a document issued by Quality Assurance that confirms that a regulated product meets its product specification. They commonly contain the actual results obtained from testing performed as part of quality control of an individual batch of a product.
How do I get a certificate of analysis?
Navigate or search for a product page.
Select the “Documentation” tab.
Click “Certificates of Analysis”
Provide the exact Lot Number.
Click “Retrieve COA”
If there is not one available, you will be given the option to request it.
What is a certificate of analysis for food?
A Certificate of Analysis (COA) is a document from a supplier that states the identity, purity or microbiological state of a product. It shows that the supplier completed the required testing and that the results meet the product specifications.
What is certificate of analysis Pharmaceutical?
Model certificate of analysis. It has been recommended in various for a that WHO should establish a model certificate of analysis for use in trade in starting materials and by manufacturers of pharmaceutical substances, recipients and medicinal products. A model of such a certificate is shown in Appendix 1.
Is a certificate of analysis a legal document?
Definition of Certificate of Analysis. Certificate of Analysis means a document, signed by an authorized representative of Manufacturer, describing Specifications for, and testing methods applied to, Product, and the results thereof.
What is certificate of analysis for food?
3.2 Certificate of Analysis. A Certificate of Analysis (COA) is a document from a supplier that states the identity, purity or microbiological state of a product. It shows that the supplier completed the required testing and that the results meet the product specifications.
What is a certificate of analysis FDA?
A Certificate of Analysis refers to an authenticated document that is issued by Illumine's Quality Assurance Department that ascertains that a product has met its predetermined product release specification(s) and quality.
What is a COA in the food industry?
A certificate of analysis (COA) is the supplier's test results on the specific lot being provided to you. Before requiring a COA, determining the key characteristics that can fluctuate, past concerns, and compliance to specifications is essential to your product or process.
What is certificate of analysis for cosmetics?
Cosmetic & Skincare Testing at AEMTEK is an ISO 17025 accredited laboratory that provides a complete Cosmetic and Skincare microbiological testing panel based on recommendations from the FDA for testing cosmetic and skincare products.
#1 usability according to G2
Try the PDF solution that respects your time.






