Categorize Link Accreditation For Free
Drop document here to upload
Up to 100 MB for PDF and up to 25 MB for DOC, DOCX, RTF, PPT, PPTX, JPEG, PNG, JFIF, XLS, XLSX or TXT
Note: Integration described on this webpage may temporarily not be available.
0
Forms filled
0
Forms signed
0
Forms sent
Discover the simplicity of processing PDFs online

Upload your document in seconds

Fill out, edit, or eSign your PDF hassle-free

Download, export, or share your edited file instantly
Top-rated PDF software recognized for its ease of use, powerful features, and impeccable support






Every PDF tool you need to get documents done paper-free
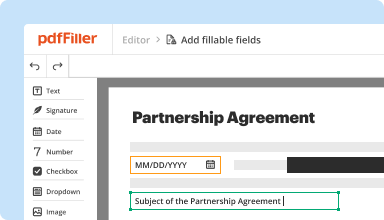
Create & edit PDFs
Generate new PDFs from scratch or transform existing documents into reusable templates. Type anywhere on a PDF, rewrite original PDF content, insert images or graphics, redact sensitive details, and highlight important information using an intuitive online editor.
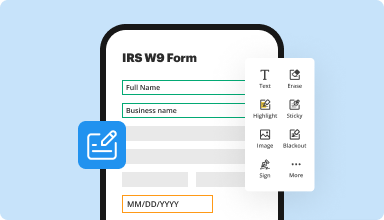
Fill out & sign PDF forms
Say goodbye to error-prone manual hassles. Complete any PDF document electronically – even while on the go. Pre-fill multiple PDFs simultaneously or extract responses from completed forms with ease.
Organize & convert PDFs
Add, remove, or rearrange pages inside your PDFs in seconds. Create new documents by merging or splitting PDFs. Instantly convert edited files to various formats when you download or export them.
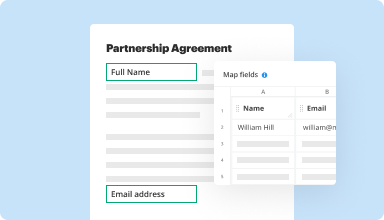
Collect data and approvals
Transform static documents into interactive fillable forms by dragging and dropping various types of fillable fields on your PDFs. Publish these forms on websites or share them via a direct link to capture data, collect signatures, and request payments.
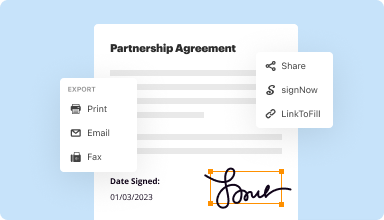
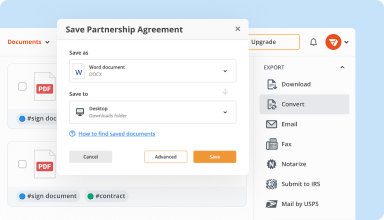
Export documents with ease
Share, email, print, fax, or download edited documents in just a few clicks. Quickly export and import documents from popular cloud storage services like Google Drive, Box, and Dropbox.
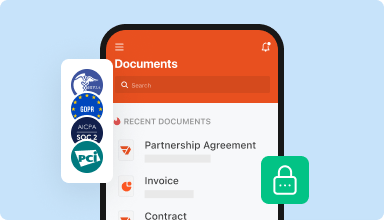
Store documents safely
Store an unlimited number of documents and templates securely in the cloud and access them from any location or device. Add an extra level of protection to documents by locking them with a password, placing them in encrypted folders, or requesting user authentication.
Customer trust by the numbers
64M+
users worldwide
4.6/5
average user rating
4M
PDFs edited per month
9 min
average to create and edit a PDF
Join 64+ million people using paperless workflows to drive productivity and cut costs
Why choose our PDF solution?
Cloud-native PDF editor
Access powerful PDF tools, as well as your documents and templates, from anywhere. No installation needed.
Top-rated for ease of use
Create, edit, and fill out PDF documents faster with an intuitive UI that only takes minutes to master.
Industry-leading customer service
Enjoy peace of mind with an award-winning customer support team always within reach.
What our customers say about pdfFiller
See for yourself by reading reviews on the most popular resources:
Worked well. Looks like a good program. I was only using it for one foerm, and do not feel I need the program. You charged me for a full year $72.00, when I felt I WASA SUBSCRIBING FOR ONE MONEH. PLEASE REFUND TTHE DIFFERENCE.
2017-04-07
Mostly I am thrilled with this service I didn't know I needed... until this week... and I needed it twice!! The form MC 030 was glitchy when it came to cut and pasting and editing the comments section. My only complaint.
2017-08-04
What do you like best?
I love that it is easy and user friendly.
What do you dislike?
I do wish there were more editing options to use.
What problems are you solving with the product? What benefits have you realized?
Easy to fill in forms
I love that it is easy and user friendly.
What do you dislike?
I do wish there were more editing options to use.
What problems are you solving with the product? What benefits have you realized?
Easy to fill in forms
2019-02-25
PDF Filler is a must for me!
I love it! As a small business owner who strives to maintain a professional business image (website, business cards, etc.) , PDFfiller does just that for the proposals I create for my customers ... both future, and repeat. I know for a fact my customers appreciate the quality and professionalism of the proposals they receive from me - they've told me so! Thanks PDFfiller!
I love the ability to quickly, and easily, create/copy professional proposals for my customers. I then save each file as a PDF (with my logo embedded at the top left of each document), and attach via e-mail for completing my estimation process with each customer.
The only thing I've discovered is with the particular template I use for my particular proposals - my template has an area that indicates "Page 1 of _" ... well, there are no additional pages that are affiliated with my template, so I end up either having to re-edit to ensure my entire proposal fits into "Page 1", or I have to continue additional pages outside of PDFfiller.
2019-01-22
Works great, I have never had a problem.
Easy to fill in forms.
Easy to use. Everything is labeled well so you can find what you need to do quick. The files are always where I need them to be.
I haven't had any problems using this software. There isn't anything bad I can say because I haven't ran into anytjing.
2017-11-14
Life saver. Worked on an assignment for hours only to be unsuccessful, found pdf filler and my assignment was not only a success within less then an hr but presentation was all around better.
2024-02-23
Intuitive and easy to use
Easily and quickly fill pdfs with this simple software, just be aware there is no free option other than a 30-day trial.
This software is really straightforward and easy to use. I find it intuitive and am able to edit pdfs quickly and painlessly. Adding a signature is really easy as well.
I did the free trial of this software and even though I was impressed, I didn't end up purchasing the monthly subscription due to my needs. There are options out there that are free, although they're definitely inferior in terms of functionality and user interface. So I think the cost (even though it is reasonable) ended up being not worth it for me specifically in the end. Other than that, I had no problems with the software and would recommend it to someone who uses pdf software frequently.
2022-02-22
its pretty user friendly even for an…
its pretty user friendly even for an old fart like me.. gets the job done and has plenty of neat features
2021-10-02
It's great
It's great. Not as good as adobe in the areas of text replacement or image addition. It is also not as sophisticated for selection mechanisms. If you need to fill in documents or create PDFs this is a solid application that can do the stuff that you will need quickly and cheaply.
2020-05-13
Categorize Link Accreditation Feature
The Categorize Link Accreditation feature helps you manage and verify the quality of links effectively. By categorizing each link, you can maintain control over your digital resources and ensure that they meet your standards.
Key Features
Link categorization for easy management
Accreditation verification process
User-friendly dashboard for quick access
Reporting tools to track link performance
Integration with other tools for seamless workflow
Potential Use Cases and Benefits
Improve link quality in marketing campaigns
Ensure compliance with industry regulations
Make informed decisions based on link performance data
Enhance user experience by providing reliable resources
Boost your site's authority with verified links
This feature addresses your need for link management by providing a structured approach to categorize and verify links. With Categorize Link Accreditation, you can identify quality links, reduce risks associated with unreliable resources, and enhance the credibility of your content. Embrace efficiency and clarity in your link management.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What if I have more questions?
Contact Support
What does it mean to be CIA certified?
The Clinical Laboratory Improvement Amendments (CIA) regulate laboratory testing and require clinical laboratories to be certificated by their state as well as the Center for Medicare and Medicaid Services (CMS) before they can accept human samples for diagnostic testing.
What is CIA and what is its purpose?
In general terms, the CIA regulations establish quality standards for laboratory testing performed on specimens from humans, such as blood, body fluid and tissue, for the purpose of diagnosis, prevention, or treatment of disease, or assessment of health.
What is the definition of CIA?
The Clinical Laboratory Improvement Amendments (CIA) of 1988 are United States federal regulatory standards that apply to all clinical laboratory testing performed on humans in the United States, except clinical trials and basic research.
Who needs a CIA certificate?
CIA requires that any facility examining human specimens for diagnosis, prevention, treatment of a disease or for assessment of health must register with the federal Centers for Medicare & Medicaid Services (CMS) and obtain CIA certification.
What does CIA exempt mean?
As defined by CIA, waived tests are simple tests with a low risk for an incorrect result. They include: Certain tests listed in the CIA regulations. ... Tests that the manufacturer applies to the FDA for waived status by providing scientific data that verifies that the CIA waiver criteria have been met.
What's the purpose of Clinical Laboratory Improvement Amendments CIA)?
The Clinical Laboratory Improvement Amendments of 1988 (CIA) regulations include federal standards applicable to all U.S. facilities or sites that test human specimens for health assessment or to diagnose, prevent, or treat disease.
How do I get CIA certified?
You can enroll your laboratory in the CIA program by completing an application (Form CMS-116) available on the CMS CIA website or from your local State Agency. Send your completed application to the address of the local State Agency for the State in which your laboratory is located.
What is a CIA certificate?
The Clinical Laboratory Improvement Amendments (CIA) regulate laboratory testing and require clinical laboratories to be certificated by their state as well as the Center for Medicare and Medicaid Services (CMS) before they can accept human samples for diagnostic testing.
How long does it take to get a CIA certificate?
How long will it take to receive our CIA certificate of waiver? Depending on your state health department, it can take between 4-12 weeks to receive your CIA certificate.
Do I need CIA certification?
Yes, there are some testing exceptions that do not require CIA certification. ... Testing that is used to gather evidence for legal purposes, and is not performed for purposes of clinical treatment, medical diagnosis, health assessment or disease prevention is not subject to CIA.
#1 usability according to G2
Try the PDF solution that respects your time.






